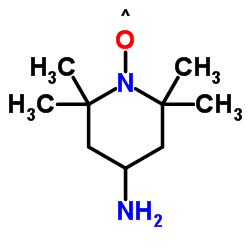
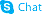4-Acetamido-TEMPO, free radical
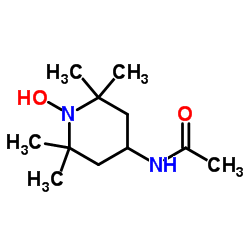
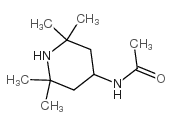
4-Acetamido-TEMPO, free radical structure
|
Common Name | 4-Acetamido-TEMPO, free radical | ||
|---|---|---|---|---|
| CAS Number | 14691-89-5 | Molecular Weight | 213.297 | |
| Density | N/A | Boiling Point | 368.8ºC at 760 mmHg | |
| Molecular Formula | C11H21N2O2 | Melting Point | 143-145 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 176.8ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 4-acetamido-tempo |
|---|---|
| Synonym | More Synonyms |
| Boiling Point | 368.8ºC at 760 mmHg |
|---|---|
| Melting Point | 143-145 °C(lit.) |
| Molecular Formula | C11H21N2O2 |
| Molecular Weight | 213.297 |
| Flash Point | 176.8ºC |
| Exact Mass | 213.160309 |
| PSA | 32.34000 |
| LogP | 1.81840 |
| Vapour Pressure | 1.9E-06mmHg at 25°C |
| Index of Refraction | 1.506 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
|
~99% 
4-Acetamido-TEM... CAS#:14691-89-5 |
| Literature: Bobbitt, James M. Journal of Organic Chemistry, 1998 , vol. 63, # 25 p. 9367 - 9374 |
|
~98% 
4-Acetamido-TEM... CAS#:14691-89-5 |
| Literature: Ma, Zhenkun; Bobbitt, James M. Journal of Organic Chemistry, 1991 , vol. 56, # 21 p. 6110 - 6114 |
|
~% 
4-Acetamido-TEM... CAS#:14691-89-5 |
| Literature: Monatshefte fur Chemie, , vol. 134, # 6 p. 843 - 850 |
|
~94% 
4-Acetamido-TEM... CAS#:14691-89-5 |
| Literature: Kashparova; Kagan; Kashparov; Zhukova Russian Journal of Applied Chemistry, 2002 , vol. 75, # 4 p. 667 - 668 |
|
~% 
4-Acetamido-TEM... CAS#:14691-89-5 |
| Literature: Carbohydrate research, , vol. 89, # 2 p. 211 - 220 |
|
Tempace and troxyl-novel synthesized 2,2,6,6-tetramethylpiperidine derivatives as antioxidants and radioprotectors.
Biochem. Mol. Biol. Int. 40(6) , 1211-9, (1996) Two novel 2,2,6,6-tetramethylpiperidine derivatives (Tempace and Troxyl) were synthesized and their capacity to act as scavengers of superoxide, inhibitors of iron and ascorbate-driven Fenton reaction... |
|
|
Rutoxyl [rutin/4-acetamide-1-hydroxy-2,2,6,6-tetramethylpiperidinium] is a new member of the class of semi-natural products of high pharmacological potency.
Biochem. Mol. Biol. Int. 42(6) , 1261-70, (1997) A novel complex, Rutoxyl [rutin/4-acetamide-1-hydroxy-2,2,6,6-tetramethylpiperidinium] was synthesized and its structure and anticancer activity were investigated. The results reported here are consis... |
|
|
Different effectiveness of piperidine nitroxides against oxidative stress induced by doxorubicin and hydrogen peroxide.
Cell Biol. Toxicol. 24(1) , 101-12, (2008) The piperidine nitroxides Tempamine and Tempace have been studied for their effect on doxorubicin (DOX) and hydrogen peroxide (H(2)O(2)) cytotoxicity in immortalized B14 cells, a model for neoplastic ... |
| 4-acetmamido-TEMPO |
| 4-Acetamido-TEMPO, free radical |
| 4-Acetamido-2,2,6,6-tetramethylpiperidinyloxy |
| 4-acetylamino 2,2,6,6-tetramethylpiperidin-N-oxyl |
| 4-Acetamido-2,2,6,6-tetramethylpiperidinooxy |
| tempace |
| 1-Piperidinyloxy, 4-(acetylamino)-2,2,6,6-tetramethyl- |
| T6NJ AO B1 B1 DMV1 F1 F1 &&Free radical |
| 4-Acetylamino-2,2,6,6-tetramethylpiperidin-1-oxyl |
| 4-Acetamido-2,2,6,6-tetramethylpiperidine 1-oxyl |
| (4-Acetamido-2,2,6,6-tetramethyl-1-piperidinyl)oxidanyl |
| (4-Acetamido-2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl |
| ACETAMINO-TEMPO |
| EINECS 423-840-3 |
| 4-ACETAMIDO-2,2,6,6-TEMPO |
| MFCD00043593 |
| 4-Acetamido-2,2,6,6-tetramethylpiperidine 1-oxyl free radical |
| 4-N-acetyl-amino-TEMPO |
| 4-acetamide-TEMPO |
| 4-acetamidoTEMPO |
| 4-Acetylamino-2,2,6,6-tetramet |
| 4-Acetamido-2,2,6,6-tetramethyl-1-piperidinyloxy, free radical |
| 4-Acetamido-TEMPO Free Radical |
| 4-Acetamino-Tempo |
| 4-ACETAMIDO-2,2,6,6-TETRAMETHYL-N-PIPERI |