| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Dimethyl but-2-ynedioate
CAS:762-42-5 |
|
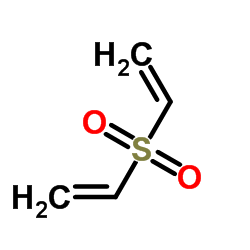 |
Divinyl sulfone
CAS:77-77-0 |
|
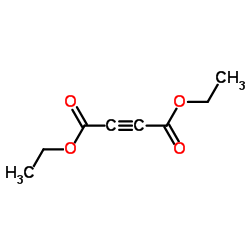 |
diethylbut-2-indioat
CAS:762-21-0 |