diethylbut-2-indioat
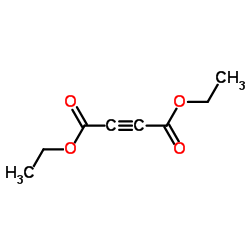
diethylbut-2-indioat structure
|
Common Name | diethylbut-2-indioat | ||
|---|---|---|---|---|
| CAS Number | 762-21-0 | Molecular Weight | 170.163 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 233.9±0.0 °C at 760 mmHg | |
| Molecular Formula | C8H10O4 | Melting Point | 1-3 °C | |
| MSDS | Chinese USA | Flash Point | 94.4±0.0 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Enhanced toxicity of the protein cross-linkers divinyl sulfone and diethyl acetylenedicarboxylate in comparison to related monofunctional electrophiles.
Chem. Res. Toxicol. 24(9) , 1457-9, (2011) Previously, we determined that diethyl acetylenedicarboxylate (DAD), a protein cross-linker, was significantly more toxic than analogous monofunctional electrophiles. We hypothesized that other protein cross-linkers enhance toxicity similarly. In agreement wi... |
|
|
3,4,5-Trisubstituted Furan-2(5H)-one Derivatives: Efficient one-pot Synthesis and Evaluation of Cytotoxic Activity.
Drug Res. (Stuttg.) , (2014) A series of 3,4,5-trisubstituted 2(5H)-furanone derivatives was synthesized through one-pot reaction of amines, aldehydes and diethyl acetylenedicarboxylate. Silica sulfuric acid efficiently catalyzes the 3-component reaction to afford the corresponding 2(5H)... |
|
|
Diels-Alder reactivity of polycyclic aromatic hydrocarbon bay regions: implications for metal-free growth of single-chirality carbon nanotubes.
J. Am. Chem. Soc. 131(44) , 16006-7, (2009) A soluble bisanthene derivative, 4,11-dimesitylbisanthene, has been synthesized in three steps from bianthrone. In hot toluene, this bisanthene undergoes a clean Diels-Alder reaction with diethyl acetylenedicarboxylate to give a rearomatized 1:1 cycloadduct a... |
|
|
Synthesis of novel highly functionalized 4-thiazolidinone derivatives from 4-phenyl-3-thiosemicarbazones.
Molecules 19(3) , 3068-83, (2014) We present herein the synthesis in good yields of two series of highly functionalized thiazolidinone derivatives from the reactions of various 4-phenyl-3-thio-semicarbazones with ethyl 2-bromoacetate and diethyl acetylenedicarboxylate, respectively. |
|
|
Synthesis of the anti-virus compound shuangkangsu's analogs.
J. Asian Nat. Prod. Res. 11(7) , 613-20, (2009) Four novel cyclic peroxide glucosides 15a, 15b, 16a, and 16b, optically pure analogs of shuangkangsu (1), which is an anti-virus natural product with an unusual skeleton isolated from the buds of Lonicera japonica Thunb, were first synthesized totally in six ... |