| Structure | Name/CAS No. | Articles |
|---|---|---|
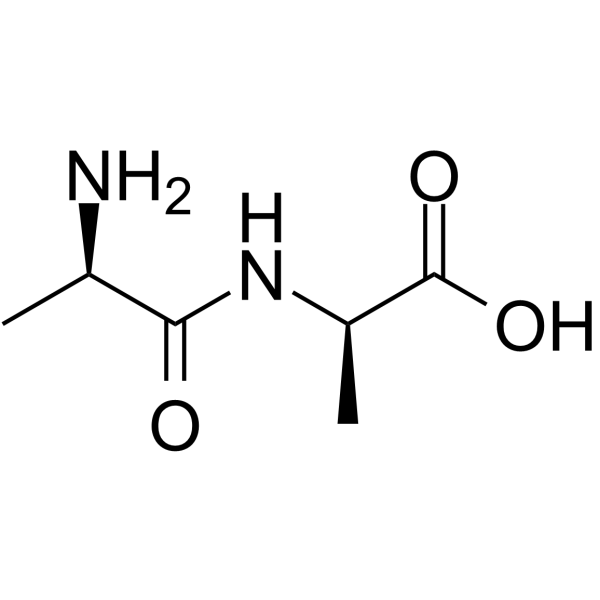 |
H-D-Ala-D-Ala-OH
CAS:923-16-0 |
|
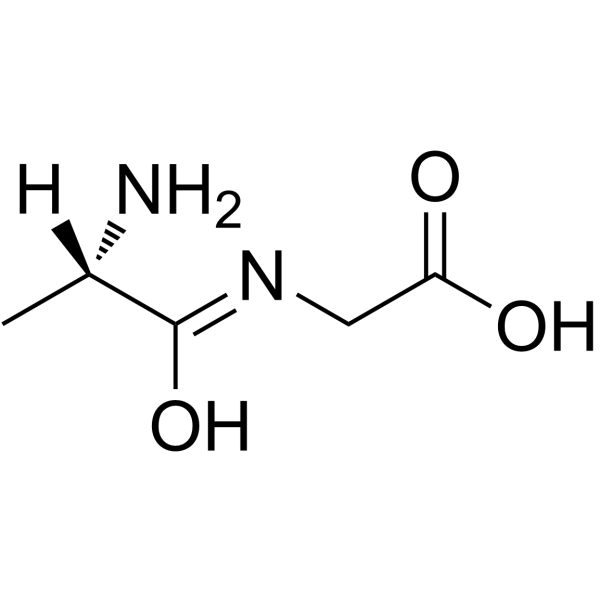 |
h-ala-gly-oh
CAS:687-69-4 |
|
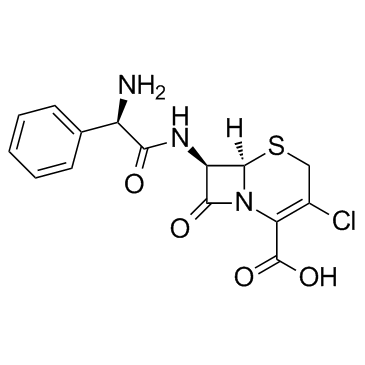 |
cefaclor
CAS:53994-73-3 |
|
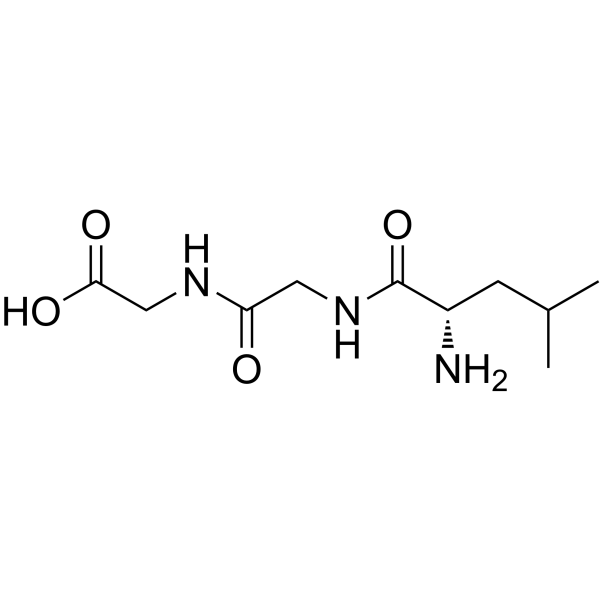 |
H-Leu-Gly-Gly-OH
CAS:1187-50-4 |
|
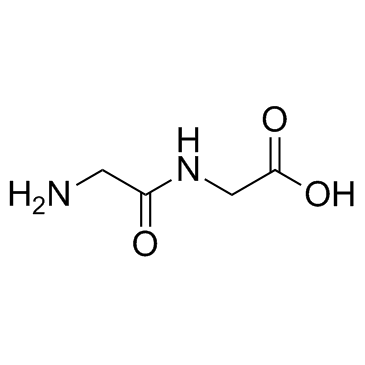 |
H-Gly-Gly-OH
CAS:556-50-3 |
|
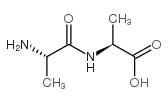 |
H-Ala-Ala-OH
CAS:1948-31-8 |
|
 |
Ampicillin
CAS:69-53-4 |