[Study of the bioequivalence of a new isosorbide dinitrate tablet formulation compared with the standard preparation].
J E Metzner, D Buchberger, N Häring, J Läuter
Index: Arzneimittelforschung 47(6) , 719-26, (1997)
Full Text: HTML
Abstract
An investigation in the bioequivalence of a new tablet formulation with 5 mg isosorbide dinitrate (CAS 87-33-2, ISDN 5 von ct) was performed in a two-way cross-over study with 18 subjects. The relative bioavailability with respect to a reference preparation for AUC related to isosorbide dinitrate was 107.5% and for Cmax 112.5%. A positive decision for bioequivalence derived from the usual confidence intervals for both parameters related to isosorbide dinitrate and the metabolites isosorbide-2-nitrate and isosorbide-5-nitrate, respectively. The difference in tmax showed no clinical relevance. The new formulation was bioequivalent to the reference.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
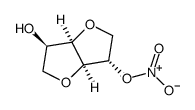 |
isosorbide 2-mononitrate
CAS:16106-20-0 |
C6H9NO6 |
|
Effects of isosorbide-5-mononitrate and isosorbide-2-mononit...
1991-01-01 [Arch. Int. Pharmacodyn. Ther. 312 , 104-9, (1991)] |
|
Pharmacokinetic/pharmacodynamic relationship of the duration...
1992-05-01 [J. Pharmacol. Exp. Ther. 261(2) , 692-700, (1992)] |
|
Concentrations of isosorbide dinitrate, isosorbide-2-mononit...
1990-01-01 [Eur. J. Clin. Pharmacol. 38(2) , 145-7, (1990)] |
|
A pharmacokinetic model for isosorbidedinitrate, isosorbide-...
1990-01-01 [Drug Metab. Dispos. 18(4) , 429-34, (1990)] |
|
Pharmacokinetics of stereomeric 1,4:3,6-dianhydrohexitol mon...
1993-01-01 [Pharm. Res. 10(1) , 22-7, (1993)] |