A pharmacokinetic model for isosorbidedinitrate, isosorbide-2-mononitrate, and isosorbide-5-mononitrate.
D Smith, W Aldrich, M Dey, R Enever, R Warner, R Weierstall
Index: Drug Metab. Dispos. 18(4) , 429-34, (1990)
Full Text: HTML
Abstract
A pharmacokinetic model is proposed to describe the plasma levels of isosorbidedinitrate (ISDN) and its two pharmacologically active metabolites, isosorbide-2-mononitrate (IS-2MN) and isosorbide-5-mononitrate (IS-5MN), following the oral administration of several 20-mg sustained release formulations of ISDN. Absorption of ISDN from the gastrointestinal tract appears first-order. A three compartment model is used to describe ISDN systemic plasma levels with t1/2 alpha = 7 min, t1/2 beta = 48 min and t1/2 gamma = 7.5 hr. The long t1/2 gamma is due to the slow release of ISDN from a peripheral compartment. ISDN undergoes extensive first-pass hepatic metabolism to IS-2MN and IS-5MN. The metabolic pathways appear to be close to saturation at an ISDN dose of 20 mg. Both IS-2MN and IS-5MN systemic plasma levels can be described by one compartment models with first-order elimination (respective elimination half-lives are 1.9 and 5.1 hr). The central compartment volumes of distribution for ISDN, IS-2MN and IS-5MN (116, 57, and 38 liters, respectively) are in agreement with reported literature values. This model is of particular usefulness as a formulation tool in designing sustained release ISDN formulations of the type investigated here since the observed first-order absorption rate constant correlates well with the in vitro first-order dissolution rate constant. Therefore, for these formulations, plasma levels can be simulated using data generated from in vitro dissolution studies, thus obviating the need for multiple human bioavailability studies.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
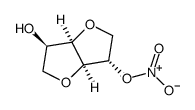 |
isosorbide 2-mononitrate
CAS:16106-20-0 |
C6H9NO6 |
|
Effects of isosorbide-5-mononitrate and isosorbide-2-mononit...
1991-01-01 [Arch. Int. Pharmacodyn. Ther. 312 , 104-9, (1991)] |
|
Pharmacokinetic/pharmacodynamic relationship of the duration...
1992-05-01 [J. Pharmacol. Exp. Ther. 261(2) , 692-700, (1992)] |
|
[Study of the bioequivalence of a new isosorbide dinitrate t...
1997-06-01 [Arzneimittelforschung 47(6) , 719-26, (1997)] |
|
Concentrations of isosorbide dinitrate, isosorbide-2-mononit...
1990-01-01 [Eur. J. Clin. Pharmacol. 38(2) , 145-7, (1990)] |
|
Pharmacokinetics of stereomeric 1,4:3,6-dianhydrohexitol mon...
1993-01-01 [Pharm. Res. 10(1) , 22-7, (1993)] |