| Structure | Name/CAS No. | Articles |
|---|---|---|
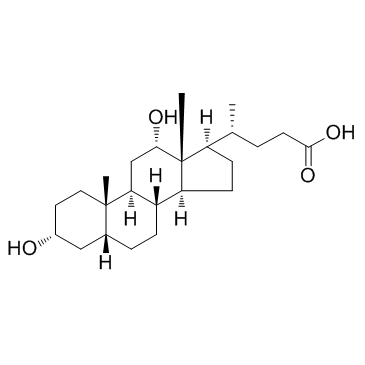 |
Deoxycholic acid
CAS:83-44-3 |
|
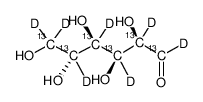 |
D-Glucose-13C6,1,2,3,4,5,6,6-d7
CAS:201417-01-8 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |