A high-performance liquid chromatography-tandem mass spectrometric method for the determination of pharmacokinetics of ganaxolone in rat, monkey, dog and human plasma.
K Ram, G N Lam, B Chien
Index: J. Chromatogr. B. Biomed. Sci. Appl. 751(1) , 49-59, (2001)
Full Text: HTML
Abstract
A method for determining concentration levels of ganaxolone in rat, monkey, dog and human plasma was validated in the range of 5-1500 ng/ml using a 200-microl plasma sample volume. This validation report describes the linearity, specificity. sensitivity, reproducibility, accuracy, recovery and stability of the analytical method. The inter-day C.V. ranged from 0.5 to 9.2%, intra-day C.V. from 0.7 to 8.8% and intra-day accuracy (mean absolute percentage difference) ranged from 0.0 to 14.0% for rat, monkey, dog and human plasma. The method was used for the routine analysis of ganaxolone in rat, monkey, dog and human plasma and summary of the pharmacokinetic data are presented.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
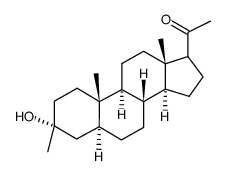 |
Ganaxolone
CAS:38398-32-2 |
C22H36O2 |
|
A quantitiative LC-MS/MS method for the measurement of arach...
2015-01-22 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 976-977 , 6-18, (2015)] |
|
Comparative evaluation of oral systemic exposure of 56 xenob...
2005-02-01 [Xenobiotica 35 , 191-210, (2005)] |
|
Endocannabinoid-mediated improvement on a test of aversive m...
2015-09-15 [Behav. Brain Res. 291 , 164-71, (2015)] |
|
A potential effect of ganaxolone in an animal model of infan...
2014-11-01 [Epilepsy Res. 108(9) , 1492-500, (2014)] |
|
Anticonvulsant doses of ganaxolone do not compromise motor p...
2010-01-29 [Neurosci. Lett. 469(3) , 396-9, (2010)] |