| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Isoniazid
CAS:54-85-3 |
|
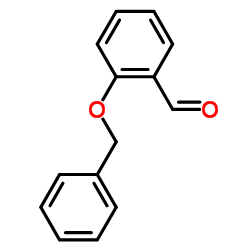 |
2-(Benzyloxy)benzaldehyde
CAS:5896-17-3 |