Computational study of khellin excited states and photobinding to DNA.
Salama Omar, Leif A Eriksson
Index: Photochem. Photobiol. Sci. 8(8) , 1179-86, (2009)
Full Text: HTML
Abstract
A theoretical investigation of the formation and spectroscopic properties of the furan and pyrone monoadducts between the photosensitizer khellin and DNA base thymine is reported. The thermal reaction pathways involve very high barriers, whereas the excited state surfaces display low barriers in regions leading to the ground state TS structures and potential wells at the ground state TS geometries. Computed UV absorption spectra are interpreted with the support of molecular orbital calculations, and the role of solvent effects on the spectra is discussed. The red-shift in the khellin spectra upon intercalation in DNA is excellently reproduced by the computational methodology, as is the solvent induced spectral shift. The data also provides an explanation to why khellin predominantly forms furan monoadducts in DNA, as opposed to the closely related psoralen compounds.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
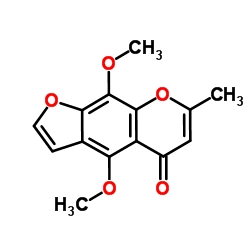 |
Khellin
CAS:82-02-0 |
C14H12O5 |
|
Validated HPLC and HPTLC methods for simultaneous determinat...
2013-03-01 [J. Chromatogr. Sci. 51(3) , 258-65, (2013)] |
|
F420H2-dependent degradation of aflatoxin and other furanoco...
2012-01-01 [PLoS ONE 7(2) , e30114, (2012)] |
|
A case study to evaluate the treatment of vitiligo with khel...
2003-01-01 [Eur. J. Dermatol. 13(5) , 474-7, (2003)] |
|
Application of 1D BIRD or X-filtered DEPT long-range C-C rel...
2006-04-01 [Magn. Reson. Chem. 44(4) , 475-80, (2006)] |
|
Determination of visnagin in rat plasma by liquid chromatogr...
2009-03-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 877(7) , 653-6, (2009)] |