A facile asymmetric synthesis of (s)-14-methyl-1-octadecene, the sex pheromone of the peach leafminer moth.
Tao Zhang, Wei-Li Ma, Tian-Rui Li, Jia Wu, Jun-Run Wang, Zhen-Ting Du
Index: Molecules 18(5) , 5201-8, (2013)
Full Text: HTML
Abstract
An asymmetric synthesis of 14-methyl-1-octadecene, the sex pheromone of the peach leafminer moth has been achieved. The target molecule was synthesized in six linear steps and in 30.3% overall yield from commercially available hexanoyl chloride, (S)-4-benzyloxazolidin-2-one and 1,9-nonanediol. The hexanoyl chloride was connected with (S)-4-benzyloxazolidin-2-one, and with the induction of the chiral oxazolidinone auxiliary, after chiral methylation, LAH reduction and then tosylation gave the chiral key intermediate 5 in high stereoselectivity. 1,9-Nonanediol, was selectively brominated, THP protected and subjected to Li₂CuCl₄-mediated C-C coupling to afford a C12 intermediate. The target molecule, (S)-14-methyl-1-octadecene, was obtained after the two parts were subjected to a second Li₂CuCl₄-mediated C-C coupling. Our synthetic approach represents the first time a substrate-control asymmetric synthesis of (S)-14-methyl-1-octadecene has been reported.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
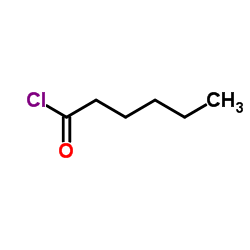 |
Hexanoyl chloride TOP1 supplier
CAS:142-61-0 |
C6H11ClO |
|
Remarkably regioselective deacylation of cellulose esters us...
2014-10-13 [Carbohydr. Polym. 111 , 25-32, (2014)] |
|
Use of multivariate statistical techniques to optimize the s...
2015-03-01 [Talanta 134 , 256-63, (2015)] |
|
One-step dispersion of cellulose nanofibers by mechanochemic...
2012-12-01 [ChemSusChem 5(12) , 2319-22, (2012)] |
|
An efficient total synthesis of ruprechstyril from Ruprechti...
2013-01-01 [Nat. Prod. Res. 27(13) , 1153-8, (2013)] |
|
Total synthesis and antibacterial screening of ( ± )-7-butyl...
2013-01-01 [J. Asian Nat. Prod. Res. 15(10) , 1112-22, (2013)] |