Journal of Pharmaceutical and Biomedical Analysis
1995-07-01
Validated capillary electrophoresis method for the assay of a range of basic drugs.
K D Altria, P Frake, I Gill, T Hadgett, M A Kelly, D R Rudd
Index: J. Pharm. Biomed. Anal. 13(8) , 951-7, (1995)
Full Text: HTML
Abstract
A capillary electrophoresis method has been developed and validated for the analysis of a wide range of basic drugs. Acceptable precision was obtained by employing an internal standard. Optimal sensitivity was obtained using low UV wavelengths. An experimentally designed study showed the method to be robust. The method has advantages over HPLC in terms of simplicity, speed and cost. The method is now in routine use for identity confirmation and assay of both drug substance and formulations.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
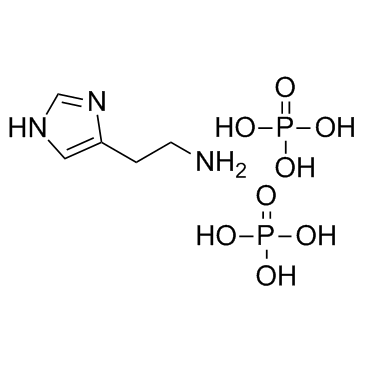 |
Histamine phosphate
CAS:51-74-1 |
C5H15N3O8P2 |
Related Articles:
More...
|
Development of a UHPLC-MS/MS method for the determination of...
2014-11-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 971 , 35-42, (2014)] |
|
Suppression of the early and late cutaneous allergic respons...
2001-01-01 [Ann. Allergy Asthma Immunol. 86(1) , 44-50, (2001)] |
|
Validated capillary electrophoretic method for the quantitat...
1996-11-08 [J. Chromatogr. B, Biomed. Appl. 686(1) , 111-7, (1996)] |
|
Nasal hyperreactivity and its effect on early and late seque...
1993-01-01 [Allergy Proc. 14(4) , 273-81, (1993)] |
|
Dose-response relationship between objective measures of his...
1991-01-01 [Acta Derm. Venereol. 71(3) , 199-204, (1991)] |