Validated capillary electrophoretic method for the quantitative analysis of histamine acid phosphate and/or benzalkonium chloride.
K D Altria, J Elgey, J S Howells
Index: J. Chromatogr. B, Biomed. Appl. 686(1) , 111-7, (1996)
Full Text: HTML
Abstract
A novel capillary electrophoresis method has been developed and validated for the quantitative determination of histamine acid phosphate (HAP) and/or benzalkonium chloride (BKC). The solutes were separated using a pH 2.5 phosphate electrolyte with detection at 200 nm. Acceptable precision was obtained using internal standardisation. The method was also acceptable for determining levels of histidine which is an impurity in HAP. Profiling of BKC homologues was demonstrated for batch identity purposes. This method is used routinely and it is intended to register this method in the British Pharmacopoeia to supplement current test methods of TLC and HPLC.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
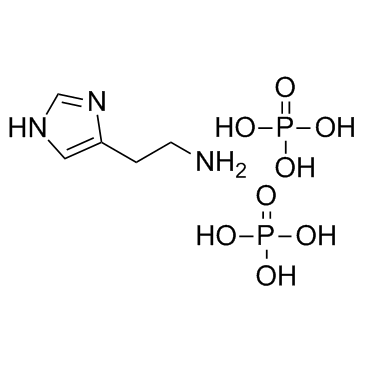 |
Histamine phosphate
CAS:51-74-1 |
C5H15N3O8P2 |
|
Development of a UHPLC-MS/MS method for the determination of...
2014-11-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 971 , 35-42, (2014)] |
|
Validated capillary electrophoresis method for the assay of ...
1995-07-01 [J. Pharm. Biomed. Anal. 13(8) , 951-7, (1995)] |
|
Suppression of the early and late cutaneous allergic respons...
2001-01-01 [Ann. Allergy Asthma Immunol. 86(1) , 44-50, (2001)] |
|
Nasal hyperreactivity and its effect on early and late seque...
1993-01-01 [Allergy Proc. 14(4) , 273-81, (1993)] |
|
Dose-response relationship between objective measures of his...
1991-01-01 [Acta Derm. Venereol. 71(3) , 199-204, (1991)] |