Ring-substituted benzohydroxamic acids: 1H, 13C and 15N NMR spectra and NH-OH proton exchange.
Jan Schraml, Marcela Tkadlecová, Statis Pataridis, Ludmila Soukupová, Vratislav Blechta, Jana Roithová, Otto Exner
Index: Magn. Reson. Chem. 43(7) , 535-42, (2005)
Full Text: HTML
Abstract
NMR spectra (1H, 13C, 15N) of para- and meta-substituted benzohydroxamic acids were studied in dry dimethyl sulfoxide solutions. The 13C chemical shifts were very close to those found by cross-polarization magic angle spinning in solids, the hydroxamic (not hydroximic) structure of which is unambiguous. The hydroxamic structure of these acids in DMSO solutions was proved independently by their 15N chemical shifts. The 15N and 1H chemical shifts of the NH-OH fragment showed excellent mutual dependences and dependences on the nature of the ring substituent. According to these dependences and ab initio energy calculations, all the acids assume the same Z conformation. Proton exchange between hydroxamic OH and NH groups in DMSO proceeded by both intra- and intermolecular exchange and the rates did not exhibit any simple relationship to the substituent constants.Copyright 2005 John Wiley & Sons, Ltd
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
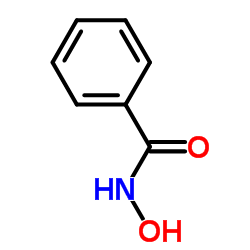 |
Benzohydroxamic acid
CAS:495-18-1 |
C7H7NO2 |
|
Multi-target spectral moment QSAR versus ANN for antiparasit...
2010-03-15 [Bioorg. Med. Chem. 18 , 2225-31, (2010)] |
|
Intermediate analogue inhibitors of mandelate racemase: N-Hy...
2007-01-01 [Bioorg. Med. Chem. Lett. 17 , 105-8, (2007)] |
|
Dephosphorylation reactions of mono-, di-, and triesters of ...
2012-12-07 [J. Org. Chem. 77(23) , 10907-13, (2012)] |
|
Optical spectra of lactoperoxidase as a function of solvent.
2005-12-06 [Biochemistry 44(48) , 15953-9, (2005)] |
|
Nanomolar inhibition of the enterobactin biosynthesis enzyme...
2006-07-15 [Bioorg. Med. Chem. Lett. 16(14) , 3802-5, (2006)] |