Benzohydroxamic acid
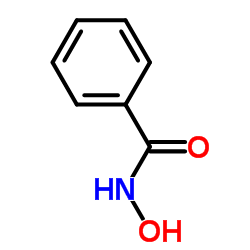
Benzohydroxamic acid structure
|
Common Name | Benzohydroxamic acid | ||
|---|---|---|---|---|
| CAS Number | 495-18-1 | Molecular Weight | 137.136 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 327.1ºC at 760 mmHg | |
| Molecular Formula | C7H7NO2 | Melting Point | 126-130 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 201ºC | |
| Name | benzohydroxamic acid |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 327.1ºC at 760 mmHg |
| Melting Point | 126-130 °C(lit.) |
| Molecular Formula | C7H7NO2 |
| Molecular Weight | 137.136 |
| Flash Point | 201ºC |
| Exact Mass | 137.047684 |
| PSA | 49.33000 |
| LogP | 0.26 |
| Index of Refraction | 1.578 |
| Stability | Moisture and Temperature Sensitive |
| Water Solubility | 22 g/L (6 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R68 |
| Safety Phrases | S45-S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DF9650000 |
| HS Code | 29280090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 29280090 |
|---|
|
Multi-target spectral moment QSAR versus ANN for antiparasitic drugs against different parasite species.
Bioorg. Med. Chem. 18 , 2225-31, (2010) There are many of pathogen parasite species with different susceptibility profile to antiparasitic drugs. Unfortunately, almost QSAR models predict the biological activity of drugs against only one pa... |
|
|
Intermediate analogue inhibitors of mandelate racemase: N-Hydroxyformanilide and cupferron.
Bioorg. Med. Chem. Lett. 17 , 105-8, (2007) Mandelate racemase (MR) catalyzes the 1,1-proton transfer that interconverts the enantiomers of mandelate. The transition state/intermediate analogues N-hydroxyformanilide (K(i)=2.79+/-0.19 microM) an... |
|
|
Dephosphorylation reactions of mono-, di-, and triesters of 2,4-dinitrophenyl phosphate with deferoxamine and benzohydroxamic acid.
J. Org. Chem. 77(23) , 10907-13, (2012) This work presents a detailed kinetic and mechanistic study of biologically interesting dephosphorylation reactions involving the exceptionally reactive nucleophilic group, hydroxamate. We compare res... |
| EINECS 207-797-6 |
| Benzohydroxamic acid |
| MFCD00002109 |
| BENZOHYROXAMICACID |
| n-hydroxy-benzamid |
| benzoylhydroxamic acid |
| BHAM |
| benzoyl hydroxylamine |
| benzohydroxamate |
| N-Hydroxy-benzamide |
| hydroxy benzamide |
| N-Hydroxybenzamide |
| Benzamide, N-hydroxy- |
| benzhydroxamic acid |
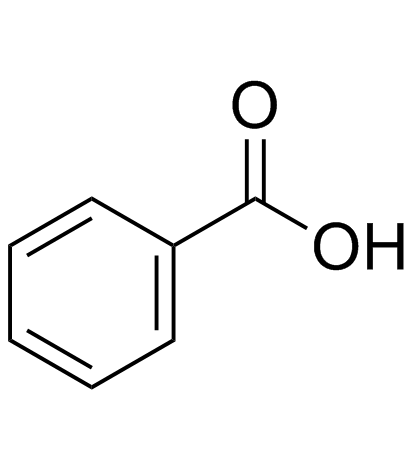 CAS#:65-85-0
CAS#:65-85-0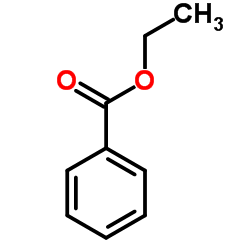 CAS#:93-89-0
CAS#:93-89-0 CAS#:98-88-4
CAS#:98-88-4 CAS#:100-52-7
CAS#:100-52-7 CAS#:932-90-1
CAS#:932-90-1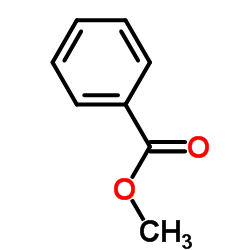 CAS#:93-58-3
CAS#:93-58-3![(1H-Benzo[d][1,2,3]triazol-1-yl)(phenyl)methanone Structure](https://image.chemsrc.com/caspic/295/4231-62-3.png) CAS#:4231-62-3
CAS#:4231-62-3 CAS#:6723-30-4
CAS#:6723-30-4 CAS#:959-22-8
CAS#:959-22-8 CAS#:106833-83-4
CAS#:106833-83-4 CAS#:107676-58-4
CAS#:107676-58-4 CAS#:491-30-5
CAS#:491-30-5 CAS#:13715-50-9
CAS#:13715-50-9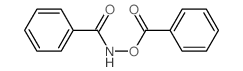 CAS#:959-32-0
CAS#:959-32-0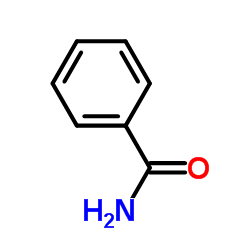 CAS#:55-21-0
CAS#:55-21-0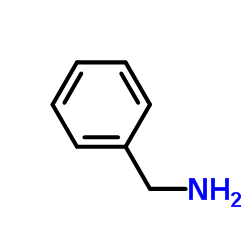 CAS#:100-46-9
CAS#:100-46-9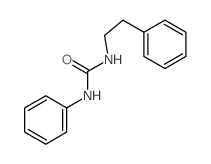 CAS#:18006-56-9
CAS#:18006-56-9
