The effect of omeprazole on the pharmacokinetics of metronidazole and hydroxymetronidazole in human plasma, saliva and gastric juice.
M J Jessa, A F Goddard, D A Barrett, P N Shaw, R C Spiller
Index: Br. J. Clin. Pharmacol. 44(3) , 245-53, (1997)
Full Text: HTML
Abstract
To evaluate the effect of omeprazole on the pharmacokinetics of metronidazole and hydroxymetronidazole in plasma, gastric juice and saliva following intravenous infusion or oral dosing of metronidazole.Eight volunteers received single doses of metronidazole (400 mg) intravenously and orally, whilst taking placebo or omeprazole (40 mg, twice daily for 5 days) in a randomized 4-way crossover study. Metronidazole and hydroxymetronidazole concentrations in plasma, saliva and gastric juice samples were determined by h.p.l.c. Pharmacokinetic parameters for metronidazole and hydroxymetronidazole were calculated, and the significance of the mean differences in parameters between omeprazole and placebo co-administration was assessed using a two-tailed, paired t-test.There were no significant differences (P < 0.05) in any of the plasma or saliva pharmacokinetic parameter values for metronidazole between volunteers receiving omeprazole or placebo when metronidazole was administered either as an intravenous infusion or orally. Following intravenous administration of metronidazole to the placebo group and omeprazole treated group respectively, the gastric transfer of metronidazole was significantly reduced from 15.5 +/- 10.4% to 2.6 +/- 1.0% of the dose (P = 0.007; 95% CI of difference 4.8 to 21.0) with concomitant changes in the metronidazole AUC (from 77.5 +/- 18.0 mumol l-1 h to 352.6 +/- 182.1 mumol l-1 h; P = 0.0003; 95% CI of difference 127.6 to 422.7), Cmax (from 61.4 +/- 26.5 mumol l-1 to 271.8 +/- 104.3 mumol l-1; p = 0.0001; 95% CI of difference 118.6 to 302.1). Similarly, the gastric juice AUC of hydroxymetronidazole was significantly reduced from 3.2 +/- 1.9 mumol l-1 h to 1.5 +/- 0.8 mumol l-1 h of the dose (P = 0.0043; 95% CI of difference 0.4 to 3.0) with a concomitant change in Cmax (from 5.0 +/- 2.5 mumol l-1 to 3.0 +/- 1.2 mumol l-1; P = 0.0007; 95% CI of difference 0.7 to 3.4).Omeprazole had little effect on the plasma and salivary pharmacokinetics of metronidazole (or its hydroxymetabolite) after intravenous or oral administration, but it did have a substantial effect on the pharmacokinetics of metronidazole and hydroxymetronidazole in gastric juice.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
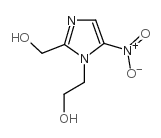 |
Hydroxymetronidazole
CAS:4812-40-2 |
C6H9N3O4 |
|
Development of a monoclonal antibody-based indirect competit...
2016-02-20 [J. Pharm. Biomed. Anal. 120 , 84-91, (2016)] |
|
Multiresidue method for the simultaneous determination of ve...
2016-04-15 [Food Chem. 197 , 571-80, (2015)] |
|
Optimized liquid-chromatographic determination of metronidaz...
1984-05-01 [Clin. Chem. 30(5) , 784-7, (1984)] |
|
Steady-state pharmacokinetics of metronidazole in Crohn's di...
1987-01-01 [Biopharm. Drug Dispos. 8(3) , 249-59, (1987)] |
|
Plasma hydroxy-metronidazole/metronidazole ratio can detect ...
1999-10-01 [Aliment. Pharmacol. Ther. 13(10) , 1335-41, (1999)] |