| Structure | Name/CAS No. | Articles |
|---|---|---|
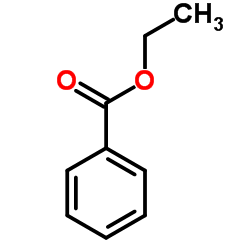 |
Ethyl benzoate
CAS:93-89-0 |
|
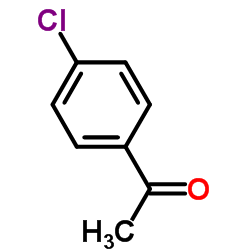 |
1-(4-Chlorophenyl)ethanone
CAS:99-91-2 |
|
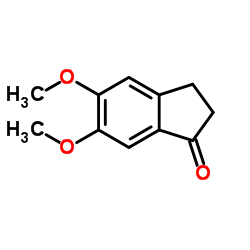 |
5,6-Dimethoxy-1-indanone
CAS:2107-69-9 |
|
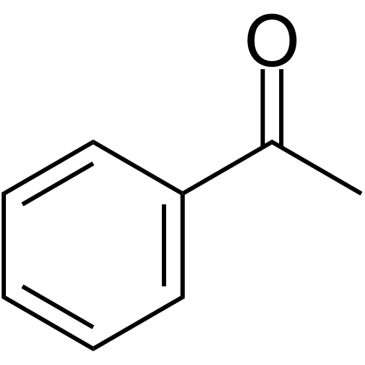 |
Acetophenone
CAS:98-86-2 |