5,6-Dimethoxy-1-indanone
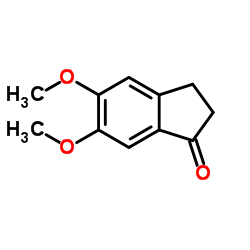
5,6-Dimethoxy-1-indanone structure
|
Common Name | 5,6-Dimethoxy-1-indanone | ||
|---|---|---|---|---|
| CAS Number | 2107-69-9 | Molecular Weight | 192.211 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 339.7±42.0 °C at 760 mmHg | |
| Molecular Formula | C11H12O3 | Melting Point | 118-122 ºC | |
| MSDS | Chinese USA | Flash Point | 150.4±14.3 °C | |
| Name | 5,6-dimethoxy-2,3-dihydroinden-1-one |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 339.7±42.0 °C at 760 mmHg |
| Melting Point | 118-122 ºC |
| Molecular Formula | C11H12O3 |
| Molecular Weight | 192.211 |
| Flash Point | 150.4±14.3 °C |
| Exact Mass | 192.078644 |
| PSA | 35.53000 |
| LogP | 2.26 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.550 |
| InChIKey | IHMQOBPGHZFGLC-UHFFFAOYSA-N |
| SMILES | COc1cc2c(cc1OC)C(=O)CC2 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914509090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Thienylhalomethylketones: Irreversible glycogen synthase kinase 3 inhibitors as useful pharmacological tools
Bioorg. Med. Chem. 17 , 6914-25, (2009) Thienylhalomethylketones, whose chemical, biological, and pharmaceutical data are here reported, are the first irreversible inhibitors of GSK-3beta described to date. Their inhibitory activity is like... |
|
|
Ring-substituted 11-oxo-11H-indeno[1,2-b]quinoline-6-carboxamides with similar patterns of cytotoxicity to the dual topo I/II inhibitor DACA.
Bioorg. Med. Chem. 7(12) , 2801-9, (1999) A series of ring-substituted analogues of the topoisomerase inhibitor 11-oxo-11H-indeno[1,2-b]quinoline-6-carboxamides was prepared and evaluated. The compounds were prepared by Pfitzinger reaction of... |
|
|
Inhibition of bovine viral diarrhea virus RNA synthesis by thiosemicarbazone derived from 5,6-dimethoxy-1-indanone.
J. Virol. 85(11) , 5436-45, (2011) In the present work, we described the activity of the thiosemicarbazone derived from 5,6-dimethoxy-1-indanone (TSC), which we previously characterized as a new compound that inhibits bovine viral diar... |
| 1H-Inden-1-one, 2,3-dihydro-5,6-dimethoxy- |
| 5,6-Dimethoxy-2,3-dihydro-1H-inden-1-on |
| EINECS 218-287-8 |
| MFCD00612461 |
| 5,6-Dimethoxy-indan-1-one |
| 1H-Inden-1-one,2,3-dihydro-5,6-dimethoxy |
| 5,6-dimethoxy-2,3-dihydro-1H-inden-1-one |
| 5,6-dimethoxyindan-1-one |
| 5,6-Dimethoxy-1-indanone |
| 3,4-dimethoxy-1-indanone |
| 5,6-Dimethoxyindan-1-on |
| 5,6-DIMETHOXY-2,3DIHYDRO-1H-INDEN-1-ONE |
| Donepezil Impurity 25 |
 CAS#:2107-70-2
CAS#:2107-70-2 CAS#:5293-43-6
CAS#:5293-43-6 CAS#:51842-87-6
CAS#:51842-87-6![5-[(3,4-dimethoxyphenyl)methyl]-2,2-dimethyl-1,3-dioxane-4,6-dione Structure](https://image.chemsrc.com/caspic/189/154317-78-9.png) CAS#:154317-78-9
CAS#:154317-78-9 CAS#:64803-82-3
CAS#:64803-82-3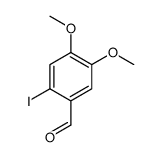 CAS#:61203-53-0
CAS#:61203-53-0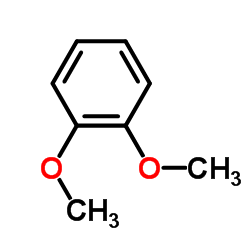 CAS#:91-16-7
CAS#:91-16-7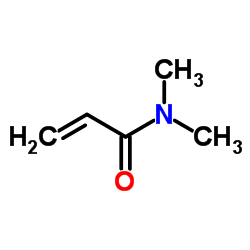 CAS#:2680-03-7
CAS#:2680-03-7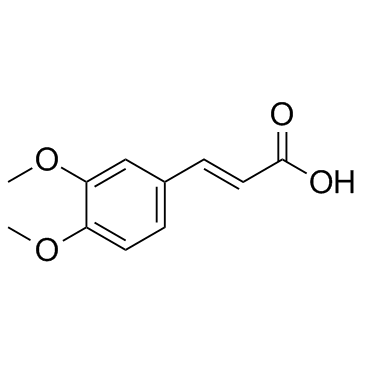 CAS#:2316-26-9
CAS#:2316-26-9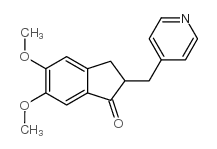 CAS#:4803-57-0
CAS#:4803-57-0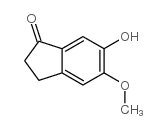 CAS#:90843-62-2
CAS#:90843-62-2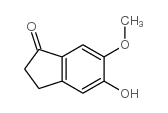 CAS#:127399-78-4
CAS#:127399-78-4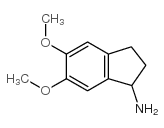 CAS#:91247-06-2
CAS#:91247-06-2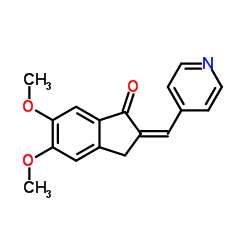 CAS#:4803-74-1
CAS#:4803-74-1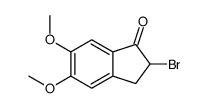 CAS#:2747-08-2
CAS#:2747-08-2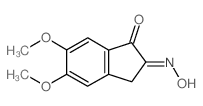 CAS#:2107-85-9
CAS#:2107-85-9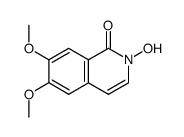 CAS#:77077-91-9
CAS#:77077-91-9![5,6-Dimethoxy-2-[(4-piperidyl)methyl]indane structure](https://image.chemsrc.com/caspic/161/844694-83-3.png) CAS#:844694-83-3
CAS#:844694-83-3 CAS#:83598-55-4
CAS#:83598-55-4
