| Structure | Name/CAS No. | Articles |
|---|---|---|
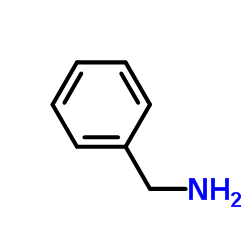 |
Benzylamine
CAS:100-46-9 |
|
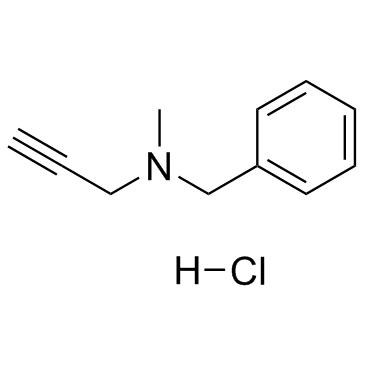 |
pargyline hydrochloride
CAS:306-07-0 |
|
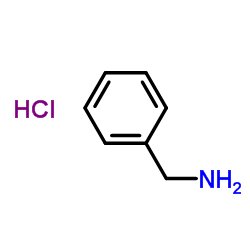 |
Benzylamine hydrochloride
CAS:3287-99-8 |
|
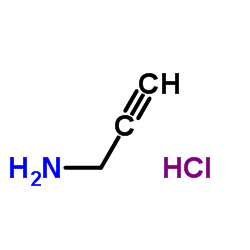 |
Prop-2-yn-1-aminium chloride
CAS:15430-52-1 |