Prop-2-yn-1-aminium chloride
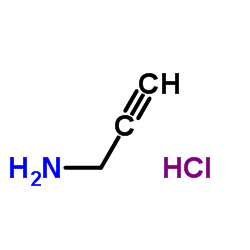
Prop-2-yn-1-aminium chloride structure
|
Common Name | Prop-2-yn-1-aminium chloride | ||
|---|---|---|---|---|
| CAS Number | 15430-52-1 | Molecular Weight | 91.539 | |
| Density | 0.989 g/mL at 25 °C(lit.) | Boiling Point | 27-28 °C(lit.) | |
| Molecular Formula | C3H6ClN | Melting Point | 179-182 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 91 °F | |
| Symbol |

GHS06 |
Signal Word | Danger | |
|
Monoclonal antibody-functionalized multilayered particles: targeting cancer cells in the presence of protein coronas.
ACS Nano 9(3) , 2876-85, (2015) Engineered particles adsorb biomolecules (e.g., proteins) when introduced in a biological medium to form a layer called a "corona". Coronas, in particular the protein corona, play an important role in determining the surface properties of particles and their ... |
|
|
Stereoselective intermolecular C-H amination reactions.
Chem. Commun. (Camb.) 48(63) , 7799-801, (2012) A novel chiral N-mesyloxycarbamate to perform rhodium-catalyzed stereoselective C-H amination reactions is reported. Chiral benzylic and propargylic amines are produced in good yields and selectivities using ethyl acetate as solvent. The corresponding free am... |
|
|
Kinetic resolution of propargylamines via a highly enantioselective non-enzymatic N-acylation process.
Chem. Commun. (Camb.) 48(85) , 10511-3, (2012) The non-enzymatic kinetic resolution of diversely substituted primary propargylic amines is reported featuring a highly selective acetyl transfer using (1S,2S)- in conjunction with Aliquat(TM) 336, affording the corresponding enantio-enriched N-acetylated pro... |
|
|
Copper(I) halide promoted diastereoselective synthesis of chiral propargylamines and chiral allenes using 2-dialkylaminomethylpyrrolidine, aldehydes, and 1-alkynes.
J. Org. Chem. 78(4) , 1463-70, (2013) Copper bromide promoted reactions of aldehydes, 1-alkynes, and chiral 2-dialkylaminomethylpyrrolidine at 25 °C give the corresponding chiral propargylamine derivatives in up to 96% yield and 99:1 dr that are readily converted to the corresponding disubstitued... |
|
|
Copper/titanium catalysis forms fully substituted carbon centers from the direct coupling of acyclic ketones, amines, and alkynes.
Angew. Chem. Int. Ed. Engl. 51(49) , 12289-92, (2012)
|
|
|
Cytotoxic betulin-derived hydroxypropargylamines trigger apoptosis.
Bioorg. Med. Chem. 21(2) , 425-35, (2013) Several novel betulin derivatives were prepared and evaluated for their antitumor activity. Among others, 3-O-acetylbetulinic aldehyde served as an ideal starting material for the synthesis of 28-acetylenic derivatives that were further transformed into Manni... |
|
|
Synthesis and biological evaluation of novel propargyl amines as potential fluorine-18 labeled radioligands for detection of MAO-B activity.
Bioorg. Med. Chem. 21(1) , 186-95, (2013) The aim of this project was to synthesize and evaluate three novel fluorine-18 labeled derivatives of propargyl amine as potential PET radioligands to visualize monoamine oxidase B (MAO-B) activity. The three fluorinated derivatives of propargyl amine ((S)-1-... |
|
|
Rapid one-pot propargylamine synthesis by plasmon mediated catalysis with gold nanoparticles on ZnO under ambient conditions.
Chem. Commun. (Camb.) 49(17) , 1732-4, (2013) Surface plasmon excitation of gold nanoparticles on ZnO in the presence of an aldehyde, an amine and phenylacetylene led to rapid and selective formation of propargylamines with good yields (50-95%) at room temperature. Plasmon mediated catalysis is the best ... |
|
|
Three-component reaction of propargyl amines, sulfonyl azides, and alkynes: one-pot synthesis of tetrasubstituted imidazoles.
Org. Lett. 14(24) , 6266-9, (2012) An efficient and straightforward strategy for the synthesis of tetrasubstituted imidazoles from propargyl amines, sulfonyl azides, and terminal alkynes is described. N-sulfonyl ketenimine and aminoallene are believed to be the key intermediates for this two-s... |
|
|
Tetrasubstituted olefinic xanthene dyes: synthesis via Pd-catalyzed 6-exo-dig cyclization/C-H activation of 2-bromobenzyl-N-propargylamines and solid state fluorescence properties.
Org. Lett. 15(2) , 382-5, (2013) Tetrasubstituted olefin based new xanthene derivatives have been synthesized via palladium-catalyzed carbopalladation/C-H activation of 2-bromobenzyl-N-propargylamine derivatives. The synthesized compounds display a pronounced solid state fluorescence due to ... |