Effect of the nature of the spacer on gene transfer efficacies of novel thiocholesterol derived gemini lipids in different cell lines: a structure-activity investigation.
Avinash Bajaj, Paturu Kondaiah, Santanu Bhattacharya
Index: J. Med. Chem. 51(8) , 2533-40, (2008)
Full Text: HTML
Abstract
A structure-activity investigation was undertaken to see the effect of the nature of the spacer on the gene transfection efficacies of thiocholesterol-derived cationic gemini lipids possessing disulfide linkage between the cationic headgroup and the thiocholesterol moiety. Three gemini cationic lipids possessing hydrophobic flexible (-(CH 2) 5-; 1), hydrophobic rigid (-C 6H 4-; 2), and hydrophilic flexible (-CH 2-CH 2-O-CH 2-CH 2-; 3) spacer segments were synthesized. In HeLa cells, lipid formulations 1 and 2 were found to be more effective as compared to lipid 3 formulation. In HT1080 cell line, the order of transfectability was 3 > 1 > 2. Transfection studies in HeLa and HT1080 cell line also showed 40-50% transfection efficacy in the presence of 10% serum conditions. These formulations were also able to transfect gene across difficult cells like HaCaT. Cytotoxic studies showed the nontoxic nature of these lipid-DNA complexes at different N/P ratios used for transfection studies.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
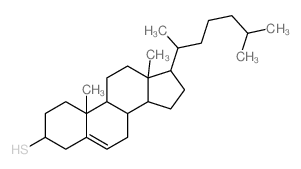 |
Cholest-5-ene-3-thiol,(3b)
CAS:1249-81-6 |
C27H46S |
|
The hedgehog receptor patched is involved in cholesterol tra...
2011-01-01 [PLoS ONE 6 , e23834, (2011)] |
|
Poly(amidoamine) conjugates with disulfide-linked cholestero...
2008-10-01 [Biomacromolecules 9(10) , 2693-704, (2008)] |
|
Thiocholesterol-based lipids for ordered assembly of bioresp...
2005-03-01 [Mol. Ther. 11(3) , 409-17, (2005)] |
|
Enhanced hepatic uptake and bioactivity of type alpha1(I) co...
2006-05-01 [J. Pharmacol. Exp. Ther. 317(2) , 797-805, (2006)] |
|
Effective incorporation of 2'-O-methyl-oligoribonucleotides ...
1992-02-11 [Nucleic Acids Res. 20(3) , 533-8, (1992)] |