Journal of Medicinal Chemistry
1985-09-01
Effects of charge, volume, and surface on binding of inhibitor and substrate moieties to acetylcholinesterase.
S G Cohen, S B Chishti, J L Elkind, H Reese, J B Cohen
Index: J. Med. Chem. 28(9) , 1309-13, (1985)
Full Text: HTML
Abstract
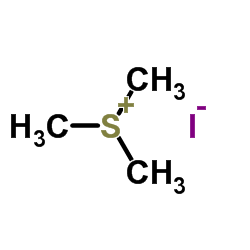
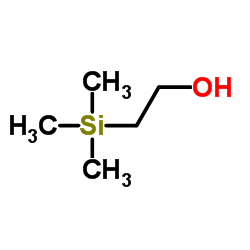
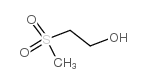
Reversible inhibitors for acetylcholinesterase, AcChE, have been studied. Sterically similar alcohols with tetra-substituted uncharged beta groups, (CH3)3SiCH2CH2OH (I), (CH3)3CCH2CH2OH (IA), and CH3S(O2)CH2CH2OH (VII), bind similarly, KI = 3-9 mM, and each binds similarly to its acetate substrate; cationic analogues, (CH3)3N+CH2CH2OH (IB) and (CH3)2S+CH2CH2OH (II), bind similarly to each other, KI = 0.4 mM, similar to Km values of their acetate substrates, and more strongly than the uncharged alcohols by approximately 1.5 kcal/mol. In comparisons of VII with CH3SO2CH3, II with (CH3)3S+, and IB with (CH3)4N+, hydroxyethyl leads to more favorable binding than methyl by approximately 0.8 kcal/mol, despite lower hydrophobicity. Two hydrophobic methyl groups, in comparison of IA with butanol, and two hydrophilic sulfone O atoms, in comparison of VII with 2-(methylthio)ethanol, increase binding similarly, by 1.0 kcal/mol. Conversion of (CH3)3S+ to (CH3)3S+O also improves binding. However, (CH3)3N+O- does not bind to AcChE, and conversion of 1-(dimethylammonio)-4-pentanone and 2-(dimethylammonio)ethyl acetate to their N-oxides, changes of identical to N+H to identical to N+--O-, decreases binding by 1.5 kcal/mol. Although the -COCH3 group in esters with well-binding beta substituents makes essentially no contribution to binding over that of the alcohols, in esters with weakly bound beta substituents, (CH3)2N+(O-), CH3N+H2, CH3S(O), CH3CH2, and CH3S binding is dominated by the ester -COCH3 group, with values of Km approximately 16 mM.