| Structure | Name/CAS No. | Articles |
|---|---|---|
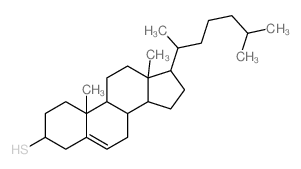 |
Cholest-5-ene-3-thiol,(3b)
CAS:1249-81-6 |
|
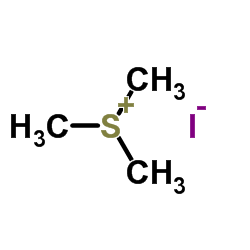 |
Trimethylsulfonium iodide
CAS:2181-42-2 |