Convergent evolution sheds light on the anti-beta -elimination mechanism common to family 1 and 10 polysaccharide lyases.
Simon J Charnock, Ian E Brown, Johan P Turkenburg, Gary W Black, Gideon J Davies
Index: Proc. Natl. Acad. Sci. U. S. A. 99(19) , 12067-72, (2002)
Full Text: HTML
Abstract
Enzyme-catalyzed beta-elimination of sugar uronic acids, exemplified by the degradation of plant cell wall pectins, plays an important role in a wide spectrum of biological processes ranging from the recycling of plant biomass through to pathogen virulence. The three-dimensional crystal structure of the catalytic module of a "family PL-10" polysaccharide lyase, Pel10Acm from Cellvibrio japonicus, solved at a resolution of 1.3 A, reveals a new polysaccharide lyase fold and is the first example of a polygalacturonic acid lyase that does not exhibit the "parallel beta-helix" topology. The "Michaelis" complex of an inactive mutant in association with the substrate trigalacturonate/Ca2+ reveals the catalytic machinery harnessed by this polygalacturonate lyase, which displays a stunning resemblance, presumably through convergent evolution, to the tetragalacturonic acid complex observed for a structurally unrelated polygalacturonate lyase from family PL-1. Common coordination of the -1 and +1 subsite saccharide carboxylate groups by a protein-liganded Ca2+ ion, the positioning of an arginine catalytic base in close proximity to the alpha-carbon hydrogen and numerous other conserved enzyme-substrate interactions, considered in light of mutagenesis data for both families, suggest a generic polysaccharide anti-beta-elimination mechanism.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
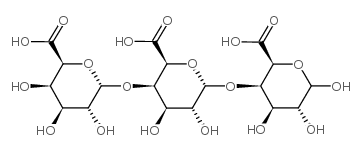 |
Trigalacturonic Acid
CAS:6037-45-2 |
C18H26O19 |
|
Molecular characterization of a thermophilic endo-polygalact...
2014-12-31 [J. Agric. Food Chem. 62(52) , 12686-94, (2015)] |
|
Oligosaccharide formation during commercial pear juice proce...
2016-08-01 [Food Chem. 204 , 84-93, (2016)] |
|
A Novel Acid-Stable Endo-Polygalacturonase from Penicillium ...
2016-06-28 [J. Microbiol. Biotechnol. 26 , 989-98, (2016)] |
|
Purification and Properties of Polygalacturonase Produced by...
2013-01-01 [Enzyme Res. 2013 , 438645, (2013)] |
|
Horizontal Gene Transfer of Pectinases from Bacteria Precede...
2016-01-01 [Sci. Rep. 6 , 26388, (2016)] |