Biodegradable star HPMA polymer conjugates of doxorubicin for passive tumor targeting.
Tomáš Etrych, Jiří Strohalm, Petr Chytil, Peter Černoch, Larisa Starovoytova, Michal Pechar, Karel Ulbrich
Index: Eur. J. Pharm. Sci. 42(5) , 527-39, (2011)
Full Text: HTML
Abstract
New biodegradable star polymer-doxorubicin (Dox) conjugates designed for passive tumor targeting were investigated and the present study described their synthesis, physico-chemical characterization, drug release and biodegradation. In the conjugates the core formed by poly(amido amine) (PAMAM) dendrimers was grafted with semitelechelic N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers bearing doxorubicin attached by hydrazone bonds, which enabled intracellular pH-controlled drug release, or by a GFLG sequence, which was susceptible to enzymatic degradation. The controlled synthesis utilizing semitelechelic copolymer precursors facilitated preparation of biodegradable polymer conjugates in a broad range of molecular weights (110-295 kDa) while still maintaining low polydispersity (∼1.7). The polymer grafts were attached to the dendrimers either through stable amide bonds or enzymatically or reductively degradable spacers, which enabled intracellular degradation of the high molecular weight polymer carrier to products that were able to be excreted from the body by glomerular filtration. Biodegradability tests showed that the rate of degradation was much faster for reductively degradable conjugates (completed within 4 h) than the degradation of conjugates linked via an enzymatically degradable oligopeptide GFLG sequence (within 72 h). This finding was likely due to the difference in steric hindrance for the small molecule glutathione and the enzyme cathepsin B. As for drug release, the conjugates were fairly stable in buffer at pH 7.4 (model of blood stream) but released doxorubicin either under mild acidic conditions or in the presence of lysosomal enzyme cathepsin B, both of which modeled the tumor cell microenvironment.Copyright © 2011 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
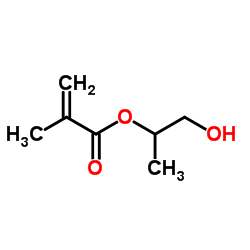 |
2-Hydroxypropyl methacrylate
CAS:27813-02-1 |
C7H12O3 |
|
Amphiphilic HPMA-LMA copolymers increase the transport of Rh...
2012-10-28 [J. Control. Release 163(2) , 170-7, (2012)] |
|
Aggregation behavior of amphiphilic p(HPMA)-co-p(LMA) copoly...
2012-12-10 [Biomacromolecules 13(12) , 4065-74, (2012)] |
|
Efficiency of high molecular weight backbone degradable HPMA...
2013-09-01 [Biomaterials 34(27) , 6528-38, (2013)] |
|
P(HPMA)-block-P(LA) copolymers in paclitaxel formulations: p...
2012-10-10 [J. Control. Release 163(1) , 63-74, (2012)] |
|
Biological activity of anti-CD20 multivalent HPMA copolymer-...
2012-03-12 [Biomacromolecules 13(3) , 727-35, (2012)] |