Journal of the American Chemical Society
2010-04-14
The productive merger of iodonium salts and organocatalysis: a non-photolytic approach to the enantioselective alpha-trifluoromethylation of aldehydes.
Anna E Allen, David W C Macmillan
Index: J. Am. Chem. Soc. 132 , 4986-4987, (2010)
Full Text: HTML
Abstract
An enantioselective organocatalytic alpha-trifluoromethylation of aldehydes has been accomplished using a commercially available, electrophilic trifluoromethyl source. The merging of Lewis acid and organocatalysis provides a new strategy for the enantioselective construction of trifluoromethyl stereogenicity, an important chiral synthon for pharmaceutical, materials, and agrochemical applications. This mild and operationally simple protocol allows rapid access to enantioenriched alpha-trifluoromethylated aldehydes through a nonphotolytic pathway.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
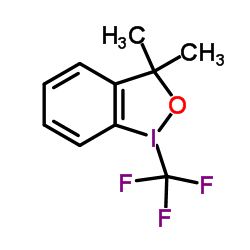 |
Togni's Reagent
CAS:887144-97-0 |
C10H10F3IO |
Related Articles:
More...
|
A Ritter-type reaction: direct electrophilic trifluoromethyl...
2011-02-01 [Angew. Chem. Int. Ed. Engl. 5th ed., 50 , 1059-1063, (2011)] |
|
Pd(II)-catalyzed ortho-trifluoromethylation of arenes using ...
2010-03-24 [J. Am. Chem. Soc. 11th ed., 132 , 3648-3649, (2010)] |
|
Copper-catalyzed trifluoromethylation of aryl and vinyl boro...
2011-05-06 [Org. Lett. 13 , 2342-2345, (2011)] |
|
Highly selective trifluoromethylation of 1,3-disubstituted a...
2012-01-09 [Angew. Chem. Int. Ed. Engl. 2nd ed., 51 , 540-543, (2012)] |
|
Direct electrophilic N-trifluoromethylation of azoles by a h...
2012-06-25 [Angew. Chem. Int. Ed. Engl. 26th ed., 51 , 6511-6515, (2012)] |