Bioorganic & Medicinal Chemistry Letters
2007-03-15
Specific stabilization of DNA triple helices by indolo[2,1-b]quinazolin-6,12-dione derivatives.
Grace Shiahuy Chen, Bhalchandra V Bhagwat, Pei-Yin Liao, Hui-Ting Chen, Shwu-Bin Lin, Ji-Wang Chern
Index: Bioorg. Med. Chem. Lett. 17(6) , 1769-72, (2007)
Full Text: HTML
Abstract
Derivatives of indolo[2,1-b]quinazolinone containing aminoalkylamino side chains were synthesized as specific DNA triplex stabilizing agents. The aminoalkylamino side chains are essential for triplex stabilization. The position-8 fluorine atom or a methyl group to the nitrogen adjacent to the planar core can enhance triplex stability by 6 degrees C and the effect is additive. Conformational analysis reveals that the orientation of the side chain underlies the ability of this compound to stabilize a DNA triplex.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
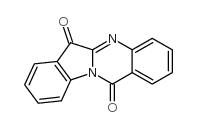 |
Tryptanthrine
CAS:13220-57-0 |
C15H8N2O2 |
Related Articles:
More...
|
Tryptanthrin ameliorates atopic dermatitis through down-regu...
2014-01-15 [Arch. Biochem. Biophys. 542 , 14-20, (2014)] |
|
Indigo naturalis and its component tryptanthrin exert anti-a...
2015-11-04 [J. Ethnopharmacol. 174 , 474-81, (2015)] |
|
Kourokhitin, a potential drug containing two active substanc...
2009-01-01 [Dokl. Biochem. Biophys. 426 , 131-3, (2009)] |
|
Inhibition of Toxoplasma gondii by indirubin and tryptanthri...
2008-12-01 [Antimicrob. Agents Chemother. 52(12) , 4466-9, (2008)] |
|
LC-APCI-MS method for detection and analysis of tryptanthrin...
2007-01-04 [J. Pharm. Biomed. Anal. 43(1) , 346-51, (2007)] |