| Structure | Name/CAS No. | Articles |
|---|---|---|
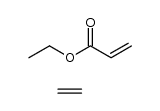 |
ethylene/ethyl acrylate copolymer
CAS:9010-86-0 |
|
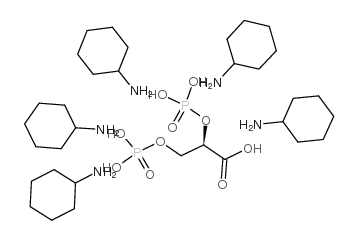 |
2,3-Diphospho-D-glyceric acid pentacyclohexylamine salt
CAS:62868-79-5 |
|
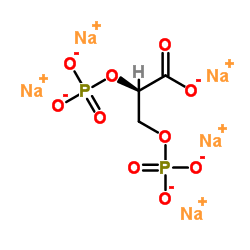 |
2,3-DIPHOSPHO-D-GLYCERIC ACID PENTASODIUM SALT
CAS:102783-53-9 |