The leucyl aminopeptidase from Helicobacter pylori is an allosteric enzyme.
Lei Dong, Ni Cheng, Ming-Wei Wang, Junfeng Zhang, Chang Shu, De-Xu Zhu
Index: Microbiology 151(Pt 6) , 2017-23, (2005)
Full Text: HTML
Abstract
This study describes the cloning, genetic analysis and biochemical characterization of a leucyl aminopeptidase (LAP) from Helicobacter pylori. A gene encoding LAP was cloned from H. pylori and the expressed 55 kDa protein displayed homology to aminopeptidases from Gram-negative bacteria, plants and mammals. This LAP demonstrated amidolytic activity against L-leucine-p-nitroanilide. Optimal activity was observed at pH 8.0 and 45 degrees C, with V(max) of 232 mumol min(-1) (mg protein)(-1) and S(0.5) of 0.65 mM. The data suggest that LAP could be allosteric (n(H)=2.27), with regulatory homohexamers, and its activity was inhibited by ion chelators and enhanced by divalent manganese, cobalt and nickel cations. Bestatin inhibited both LAP activity (IC(50)=49.9 nM) and H. pylori growth in vitro. The results point to the potential use of LAP as a drug target to develop novel anti-H. pylori agents.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
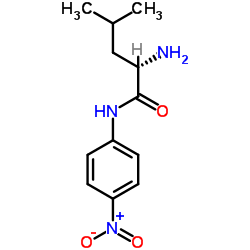 |
N-(4-Nitrophenyl)-L-leucinamide
CAS:4178-93-2 |
C12H17N3O3 |
|
Screening the Medicines for Malaria Venture "Malaria Box" ag...
2015-01-01 [PLoS ONE 10(2) , e0115859, (2015)] |
|
Beta-phenyl cysteine: a leucine aminopeptidase inhibitor.
1984-06-01 [Pharmacol. Res. Commun. 16(6) , 533-8, (1984)] |
|
Exploration of structural and physicochemical requirements a...
2013-02-01 [Mol. Divers. 17(1) , 123-37, (2013)] |
|
Covalent immobilisation of protease and laccase substrates o...
2010-08-01 [Chemosphere 80(8) , 922-8, (2010)] |
|
Aminopeptidase M from human liver. I. Solubilization, purifi...
1989-11-01 [J. Biochem. 106(5) , 818-25, (1989)] |