Stereoselective vascular effects of the (R)- and (S)-enantiomers of propranolol and atenolol.
K Stoschitzky, W Lindner, W Kiowski
Index: J. Cardiovasc. Pharmacol. 25 , 268-272, (1995)
Full Text: HTML
Abstract
All beta-adrenergic antagonists have an asymmetric carbon atom, and most commercially available beta-blockers consist of (R)- and (S)-enantiomers in a fixed 1:1-ratio. The drugs are believed to be contraindicated when peripheral vascular disease exists, presumably due to unopposed alpha-adrenergic vasoconstriction. However, little is known about direct vascular effects of beta-blockers or of stereoselective effects on peripheral arteries. Therefore, we investigated the effects on forearm blood flow (FBF) of brachial artery infusions of the (R)- and (S)- enantiomers of propranolol and atenolol (2, 10, and 50 micrograms/min each) and their inhibitory effects on isoprenaline (Iso)-induced vasodilatation by forearm venous occlusion plethysmography in 12 healthy subjects. Only (R)-propranolol caused an increase in FBF (+21%, p < 0.05), whereas (S)-propranolol and (R)- and (S)-atenolol had no direct effect on peripheral arteries. Vasodilatation induced by Iso was abolished by (S)-propranolol and reduced by (R)-propranolol (-56%, p < 0.05) and (S)-atenolol (-68%, p < 0.05), whereas (R)-atenolol had no effect. Our results indicate that the optically pure (R)- and (S)-enantiomers of propranolol and atenolol do not exert direct vasoconstrictive effects. Furthermore, our results confirm that predominantly (S)-enantiomers have beta-adrenoceptor blocking effects, but they also show that neither the non-beta-blocking (R)-enantiomer of propranolol nor the (S)-enantiomer of the beta 1-selective agent atenolol is completely devoid of blocking effects on vascular beta 2-adrenoceptors.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
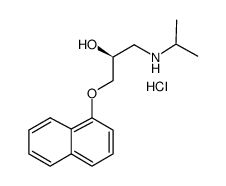 |
(S)-(-)-PROPRANOLOL HYDROCHLORIDE
CAS:4199-10-4 |
C16H22ClNO2 |
|
Chemical genetics reveals a complex functional ground state ...
2007-05-01 [Nat. Chem. Biol. 3(5) , 268-273, (2007)] |
|
Genetic mapping of targets mediating differential chemical p...
2009-10-01 [Nat. Chem. Biol. 5 , 765-71, (2009)] |
|
The Alpha-1D Is the Predominant Alpha-1-Adrenergic Receptor ...
2009-01-01 [J. Am. Coll. Cardiol. 54 , 1137-45, (2009)] |
|
A behavioural and biochemical study in mice and rats of puta...
1985-03-01 [Br. J. Pharmacol. 84 , 743-753, (1985)] |
|
Reversal of propranolol blockade of adrenergic receptors and...
1999-09-01 [Proc. Soc. Exp. Biol. Med. 221 , 382-385, (1999)] |