| Structure | Name/CAS No. | Articles |
|---|---|---|
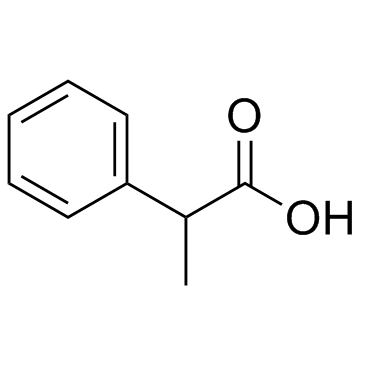 |
DL-2-Phenylpropionic acid
CAS:492-37-5 |
|
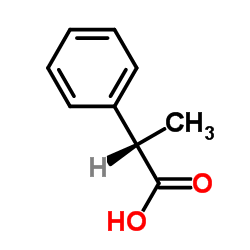 |
(S)-2-Phenylpropanoic acid
CAS:7782-24-3 |
|
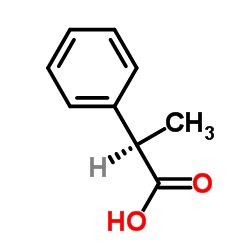 |
(R)-2-Phenylpropanoic acid
CAS:7782-26-5 |