Infrared spectroscopy study of structural changes in glass-forming salol.
J Baran, N A Davydova
Index: Phys. Rev. E. Stat. Nonlin. Soft Matter Phys. 81(3 Pt 1) , 031503, (2010)
Full Text: HTML
Abstract
We report the investigation of glass-forming salol upon different courses of the temperature changes from liquid to glass state and back using FT-IR spectroscopy measurements in the wide spectral and temperature ranges. The formation of the ordered clusters in supercooled liquid salol has been observed at 250 K. When the temperature is decreased further to 11 K these ordered clusters become an element of the glass structure. With increasing temperature to 270 K through the glass transition noticeable evolutions of the IR spectrum occurs up till the ordered clusters are developed into crystal. So produced crystal melts in the temperature range 300-310 K, that corresponds to the melting temperature of the metastable phase (Tmelt=302 K) . Thus, the crystalline structure of the ordered clusters corresponds to the structure of metastable phase and is monoclinic.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
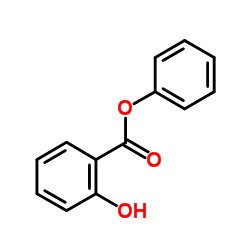 |
Phenyl salicylate
CAS:118-55-8 |
C13H10O3 |
|
Leaching of additives and degradation products from cold-cur...
1994-06-01 [Acta Odontol. Scand. 52(3) , 150-6, (1994)] |
|
In vitro transformation of hamster embryo cells by 3-(N-sali...
1981-01-01 [Toxicol. Lett. 7(3) , 211-5, (1981)] |
|
Fragrance material review on phenyl salicylate.
2007-01-01 [Food Chem. Toxicol. 45 Suppl 1 , S472-6, (2007)] |
|
Degradation kinetics of hydrolytically susceptible drugs in ...
2007-09-05 [Int. J. Pharm. 342(1-2) , 62-71, (2007)] |
|
Effect of ultraviolet light absorbers on photostabilization ...
2003-01-01 [Nat. Prod. Res. 17(1) , 21-6, (2003)] |