Analysis of tofisopam in human serum by column-switching semi-micro high-performance liquid chromatography and evaluation of tofisopam bioequivalency.
Soo Kyoung Baek, Sung Jin Choi, Jeong Soo Kim, Eun Jeon Park, Dong Hwan Sohn, Hee-Yong Lee, Hye Suk Lee
Index: Biomed. Chromatogr. 16(4) , 277-81, (2002)
Full Text: HTML
Abstract
A rapid and sensitive column-switching semi-micro HPLC method is described for the direct analysis of tofisopam in human serum. The sample (100 microL) was directly injected onto the precolumn (Capcell Pak MF Ph-1), where unretained proteins were eluted to waste. Tofisopam was then eluted into an enrichment column using 13% acetonitrile in 50 mM phosphate buffer (pH 7.0) containing 5 mM sodium octanesulfonate and subsequently into the analytical column using 43% acetonitrile in 0.1% phosphoric acid containing 5 mM sodium octanesulfonate. The detection limit (2 ng/mL), good precision (CV < or = 4.2%) and speed (total analysis time 24 min) of the present method were sufficient for drug monitoring. This method was successfully applied to a bioequivalence test of two commercial tofisopam tablets.Copyright 2002 John Wiley & Sons, Ltd.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
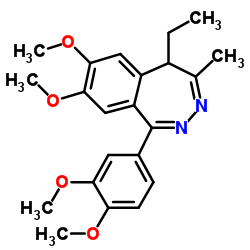 |
Tofisopam
CAS:22345-47-7 |
C18H39N5O13S |
|
The atypical anxiolytic drug, tofisopam, selectively blocks ...
2010-11-01 [J. Neural Transm. Gen. Sect. 117(11) , 1319-25, (2010)] |
|
[Pineal hormone melatonin in low doses potentiates psychotro...
2013-01-01 [Eksp. Klin. Farmakol. 76(4) , 15-7, (2013)] |
|
Clinical trial: dextofisopam in the treatment of patients wi...
2008-01-15 [Aliment. Pharmacol. Ther. 27(2) , 197-206, (2008)] |
|
Various effects of antidepressant drugs on bone microarchite...
2007-05-15 [Toxicol. Appl. Pharmacol. 221(1) , 111-8, (2007)] |
|
Method validation and determination of enantiomers and confo...
2006-09-29 [J. Chromatogr. A. 1129(1) , 47-53, (2006)] |