Influence of alkyl group on amide nitrogen atom on fluorescence quenching of tyrosine amide and N-acetyltyrosine amide.
Justyna Mrozek, Alicja Rzeska, Katarzyna Guzow, Jerzy Karolczak, Wies_aw Wiczk
Index: Biophys. Chem. 111(2) , 105-13, (2004)
Full Text: HTML
Abstract
The steady-state and time-resolved fluorescence spectroscopy was applied to determine the influence of an alkyl substituent(s) (methyl or ethyl, n-propyl, iso-propyl, n-butyl, sec-butyl, or t-butyl) on amide nitrogen atom on photophysical properties of tyrosine and N-acetyltyrosine amides in water. Generally, the amide group strongly quenches the fluorescence of tyrosine, however, the size and number of substituents on amide nitrogen atom modify the quenching process only in small degree. The fluorescence intensity decays of all amides studied are bi-exponential. The contribution of both components (alphai) to the fluorescence decay undergoes irregular change. An introduction of alkyl substituent on amide nitrogen atom causes an increase of the fluorescence lifetime of tyrosine derivative compared to the unsubstituted amide for both N-acetyltyrosine and tyrosine with the protonated amino group. Calculated, basing on the fluorescence quantum yield (QY) and average lifetime, the radiative rate constants (kf) are similar, which indicates that the substituent(s) does not have substantial influence on radiative process of the deactivation of the excited state of the phenol chromophore for all compounds studied regardless the amino group status as well as the number and type of substituent (linear or branched). The comparison of the ground-state rotamer populations of tyrosine amides and N-acetyltyrosine amides with different alkyl substituent on amide nitrogen atom obtained from 1H NMR with the value of pre-exponential factors indicates that not the rotamer populations, but specific hydration of a whole molecule of the amino acid including chromophore and amino acid moiety, seems to be the main reason of the heterogenous fluorescence intensity decay of tyrosine derivatives.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
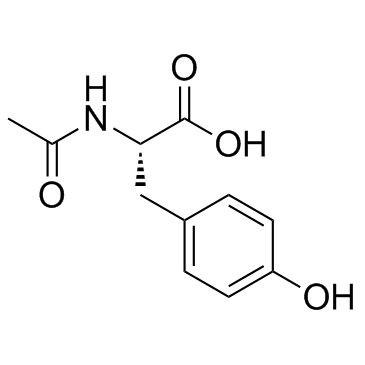 |
N-Acetyl-L-tyrosine
CAS:537-55-3 |
C11H13NO4 |
|
Surfactants, aromatic and isoprenoid compounds, and fatty ac...
2009-05-01 [Antimicrob. Agents Chemother. 53 , 1898-906, (2009)] |
|
Detection of autosomal dominant polycystic kidney disease by...
2011-06-01 [Kidney Int. 79(11) , 1244-53, (2011)] |
|
Newborn screening for congenital adrenal hyperplasia: additi...
2007-07-01 [J. Clin. Endocrinol. Metab. 92(7) , 2581-9, (2007)] |
|
Utilization of tyrosine-containing dipeptides and N-acetyl-t...
1995-04-01 [Hepatology 21(4) , 923-8, (1995)] |
|
N-Glycans protect proteins from protease digestion through t...
2000-03-01 [J. Biochem. 127(3) , 427-33, (2000)] |