N-Glycans protect proteins from protease digestion through their binding affinities for aromatic amino acid residues.
T Nishiyama, N Kimura, Y Jitsuhara, M Uchida, F Ochi, H Yamaguchi
Index: J. Biochem. 127(3) , 427-33, (2000)
Full Text: HTML
Abstract
It was previously revealed [Yamaguchi, H., Nishiyama, T., and Uchida, M. (1999) J. Biochem. 126, 261-265] that N-glycans of both the high-mannose and complex types have binding affinity for aromatic amino acid residues. This study shows that free N-glycans protect proteins from protease digestion through their binding affinities for the aromatic amino acid residues exposed on protein molecules. Protease digestion of bovine pancreatic RNase A and bovine a-lactalbumin was depressed in solutions (1 mM or so) of free N-glycans of both the high-mannose and complex types. The increasing order of the protective effects of the N-glycans paralleled that of their affinities for aromatic amino acid residues; and the presence of aromatic amino acids practically abolished the protective effects of the N-glycans. The N-glycans also depressed the protease digestion of metallothionein, an aromatic amino acid-free protein, in agreement with the observation that the N-glycans also interact with the solvent-exposed aromatic amino acid residues of the proteases. Thus it seems probable that the N-glycans protect proteins from protease digestion by steric hindrance attributable to their binding affinity for the solvent-exposed aromatic amino acid residues of both substrate proteins and proteases.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
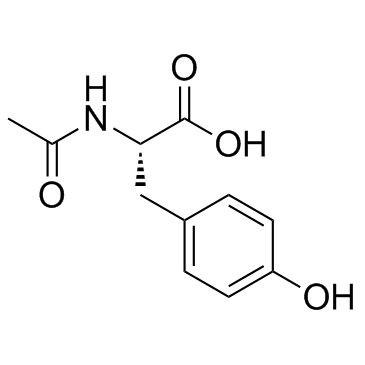 |
N-Acetyl-L-tyrosine
CAS:537-55-3 |
C11H13NO4 |
|
Surfactants, aromatic and isoprenoid compounds, and fatty ac...
2009-05-01 [Antimicrob. Agents Chemother. 53 , 1898-906, (2009)] |
|
Detection of autosomal dominant polycystic kidney disease by...
2011-06-01 [Kidney Int. 79(11) , 1244-53, (2011)] |
|
Newborn screening for congenital adrenal hyperplasia: additi...
2007-07-01 [J. Clin. Endocrinol. Metab. 92(7) , 2581-9, (2007)] |
|
Utilization of tyrosine-containing dipeptides and N-acetyl-t...
1995-04-01 [Hepatology 21(4) , 923-8, (1995)] |
|
Influence of alkyl group on amide nitrogen atom on fluoresce...
2004-09-01 [Biophys. Chem. 111(2) , 105-13, (2004)] |