Steroids
1995-06-01
Microwave-induced organic reactions of bile acids: esterification, deformylation and deacetylation using mild reagents.
B Dayal, K Rao, G Salen
Index: Steroids 60(6) , 453-7, (1995)
Full Text: HTML
Abstract
An efficient and convenient procedure for the esterification, deformylation, and deacetylation of bile acids is described. This is achieved by the addition of a catalytic amount of methanesulfonic acid or para-toluene sulfonic acid to a solution of bile acid in methanol in the domestic microwave oven. All these reactions were completed in the microwave oven within 1-3 min at 60% power (390 W) and the desired bile acids, namely trihydroxy-5 beta-cholestanoic acid, (23R)-3 alpha,7 alpha,23-trihydroxy-5 beta-cholan-24-oic acid, ursocholic acid and 7-ketolithocholic acid were isolated in 86-94% yield.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
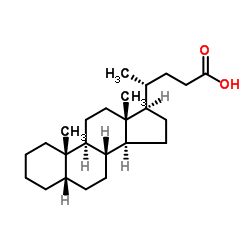 |
5β-cholanoic acid
CAS:546-18-9 |
C24H40O2 |
Related Articles:
More...
|
An efficient synthesis of 4 beta- and 6 alpha-hydroxylated b...
1993-02-01 [Steroids 58(2) , 52-8, (1993)] |
|
Calcium- and voltage-gated potassium (BK) channel activators...
2012-10-01 [ChemMedChem 7(10) , 1784-92, (2012)] |
|
Cholanic acids determined in commercial drugs by means of a ...
1993-01-01 [J. Pharm. Biomed. Anal. 11(11-12) , 1207-14, (1993)] |
|
Synthesis of [3,4-(13)c(2)]-enriched bile salts as NMR probe...
2002-09-20 [J. Org. Chem. 67(19) , 6764-71, (2002)] |
|
Synthesis of 7- and 12-hydroxy- and 7,12-dihydroxy-3-keto-5 ...
1993-11-01 [Steroids 58(11) , 524-6, (1993)] |