| Structure | Name/CAS No. | Articles |
|---|---|---|
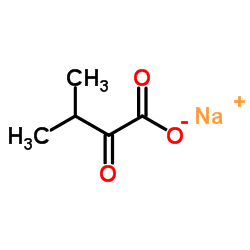 |
Sodium 3-methyl-2-oxobutanoate
CAS:3715-29-5 |
|
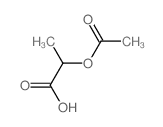 |
2-(Acetyloxy)-propanoic acid
CAS:535-17-1 |