| Structure | Name/CAS No. | Articles |
|---|---|---|
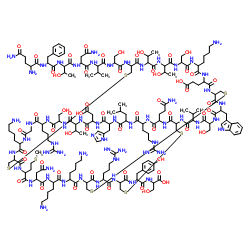 |
Charybdotoxin
CAS:95751-30-7 |
|
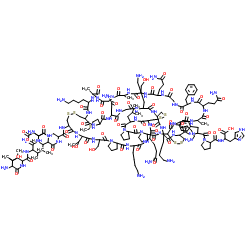 |
Margatoxin
CAS:145808-47-5 |