| Structure | Name/CAS No. | Articles |
|---|---|---|
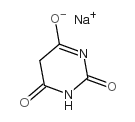 |
sodium barbiturate
CAS:4390-16-3 |
|
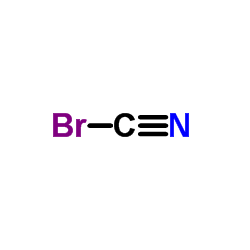 |
Cyanogen bromide
CAS:506-68-3 |