Synthesis of bromo-, boryl-, and stannyl-functionalized 1,2-bis(trimethylsilyl)benzenes via Diels-Alder or C-H activation reactions.
Christian Reus, Nai-Wei Liu, Michael Bolte, Hans-Wolfram Lerner, Matthias Wagner
Index: J. Org. Chem. 7th ed., 77 , 3518-3523, (2012)
Full Text: HTML
Abstract
1,2-Bis(trimethylsilyl)benzenes are key starting materials for the synthesis of benzyne precursors, Lewis acid catalysts, and certain luminophores. We have developed efficient, high-yield routes to functionalized 4-R-1,2-bis(trimethylsilyl)benzenes, starting from either 1,2-bis(trimethylsilyl)acetylene/5-bromopyran-2-one (2) or 1,2-bis(trimethylsilyl)benzene (1)/bis(pinacolato)diborane. In the first reaction, 5 (R = Br) is obtained through a cobalt-catalyzed Diels-Alder cycloaddition. The second reaction proceeds via iridium-mediated C-H activation and provides 8 (R = Bpin). Besides its use as a Suzuki reagent, compound 8 can be converted into 5 with CuBr(2) in i-PrOH/MeOH/H(2)O. Lithium-bromine exchange on 5, followed by the addition of Me(3)SnCl, gives 10 (R = SnMe(3)), which we have applied for Stille coupling reactions. A Pd-catalyzed C-C coupling reaction between 5 and 8 leads to the corresponding tetrasilylbiphenyl derivative. The bromo derivative 5 cleanly undergoes Suzuki reactions with electron-rich as well as electron-poor phenylboronic acids.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
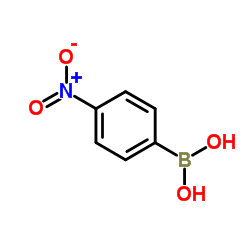 |
(4-Nitrophenyl)boronic acid
CAS:24067-17-2 |
C6H6BNO4 |
|
Ruthenium(0)-catalyzed sp3 C-H bond arylation of benzylic am...
2012-04-06 [Org. Lett. 7th ed., 14 , 1930-1933, (2012)] |
|
A new access to 3-substituted-1(2H)-isoquinolone by tandem p...
2012-04-07 [Org. Biomol. Chem. 13th ed., 10 , 2683-2691, (2012)] |
|
Copper-mediated sequential cyanation of aryl C-B and arene C...
2012-02-08 [J. Am. Chem. Soc. 5th ed., 134 , 2528-2531, (2012)] |
|
B-ring-modified isocombretastatin A-4 analogues endowed with...
2011-12-09 [ChemMedChem 12th ed., 6 , 2179-2191, (2011)] |
|
P1-substituted symmetry-based human immunodeficiency virus p...
2011-10-27 [J. Med. Chem. 54 , 7094-7104, (2011)] |