Journal of Organic Chemistry
2010-06-18
Synthesis of N-acyl-5-aminopenta-2,4-dienals via base-induced ring-opening of N-acylated furfurylamines: scope and limitations.
Cécile Ouairy, Patrick Michel, Bernard Delpech, David Crich, Christian Marazano
Index: J. Org. Chem. 75(12) , 4311-4, (2010)
Full Text: HTML
Abstract
N-Acylation of furfurylamines provided 1, which on double deprotonation with LDA led to the formation of N-acyl-5-aminopenta-2,4-dienals 2 via an isomerization involving opening of the furan ring. The scope and limitations of the procedure were examined by considering the influence of substituents on the carbonyl group and also on the heterocycle moiety. The efficacy of the reaction was shown to be very dependent on the nature of these groups.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
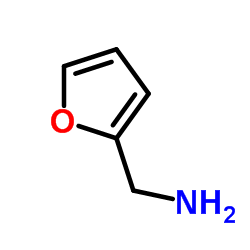 |
Furfurylamine
CAS:617-89-0 |
C5H7NO |
Related Articles:
More...
|
Biosynthesis of the 5-(Aminomethyl)-3-furanmethanol moiety o...
2014-07-22 [Biochemistry 53(28) , 4635-47, (2014)] |
|
Optimization of the Ugi reaction using parallel synthesis an...
2008-01-01 [J. Vis. Exp. (21) , doi:10.3791/942, (2008)] |
|
Induction of neurite outgrowth in 3D hydrogel-based environm...
2015-09-01 [Biomed. Mater. 10 , 051001, (2015)] |
|
Biodegradable hyaluronic acid hydrogels to control release o...
2015-11-01 [Mater. Sci. Eng. C. Mater. Biol. Appl. 56 , 311-7, (2015)] |
|
Effect of a novel chemical mixture on senescence processes a...
2001-05-01 [J. Agric. Food Chem. 49(5) , 2569-75, (2001)] |