Chirality
1998-01-01
Enantioseparation of atropisomeric 1,1'-binaphthyl-2,2'-diyl hydrogen phosphate in capillary electrophoresis by using di- and oligosaccharides as chiral selectors: di- and oligosaccharide chiral selectors in capillary electrophoresis.
B Chankvetadze, M Saito, E Yashima, Y Okamoto
Index: Chirality 10(1-2) , 134-9, (1998)
Full Text: HTML
Abstract
Twelve different disaccharides and a series of noncyclic malto- and cello-oligosaccharides were used as chiral selectors in capillary electrophoresis (CE). Most saccharides resolved the enantiomers of atropisomeric 1,1'-binaphthyl-2,2'-diyl hydrogen phosphate (BDHP) depending on the type (alpha or beta) and position of the linkage between monosaccharides. The effect of chain length of malto- and cello-oligosaccharides on enantioseparation of BDHP was also investigated. The nature of cations in background electrolytes affected significantly the separation of BDHP enantiomers.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
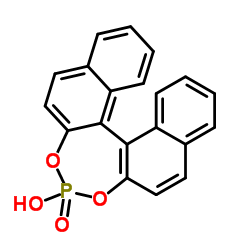 |
1,1'-Binaphthyl-2,2'-diyl hydrogenphosphate
CAS:35193-63-6 |
C20H13O4P |
Related Articles:
More...
|
Multivariate approach for the enantioselective analysis in m...
2009-01-01 [J. Chromatogr. A. 1216(5) , 845-56, (2009)] |
|
Comparison of alkylglycoside surfactants in enantioseparatio...
1997-06-01 [Electrophoresis 18(6) , 912-918, (1997)] |
|
Optimization of 12 chiral analytes with 8 polymeric surfacta...
2008-10-01 [J. Chromatogr. Sci. 46(9) , 757-63, (2008)] |
|
Use of multivariate analysis for optimization of separation ...
2006-11-01 [Electrophoresis 27(21) , 4127-40, (2006)] |
|
Chiral selectivity of guanosine media in capillary electroph...
2011-06-01 [Electrophoresis 32(13) , 1735-41, (2011)] |