| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
PROTEIN TYROSINE PHOSPHATASE SUBSTRATE
CAS:104077-19-2 |
|
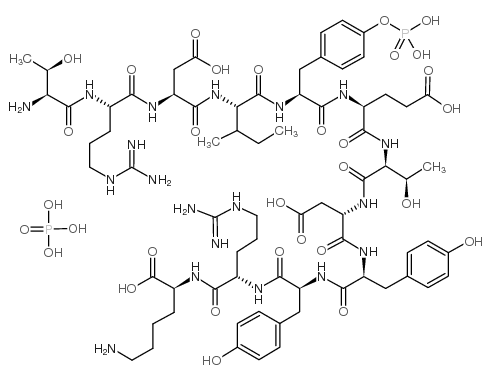 |
Protein Tyrosine Phosphatase Substrate Monophosphate
CAS:117872-62-5 |