| Structure | Name/CAS No. | Articles |
|---|---|---|
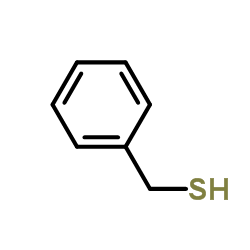 |
benzylthiol
CAS:100-53-8 |
|
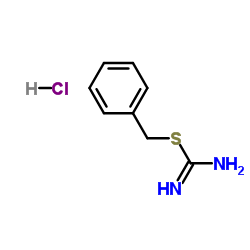 |
U 19451A
CAS:538-28-3 |
|
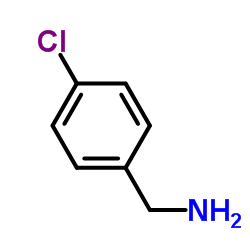 |
4-Chlorobenzylamine
CAS:104-86-9 |
|
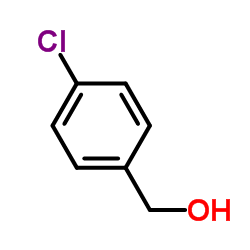 |
p-chlorobenzylalcohol
CAS:873-76-7 |