Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure.
Laura P James, Lynda Letzig, Pippa M Simpson, Edmund Capparelli, Dean W Roberts, Jack A Hinson, Timothy J Davern, William M Lee
Index: Drug Metab. Dispos. 37(8) , 1779-84, (2009)
Full Text: HTML
Abstract
Acetaminophen (APAP)-induced liver toxicity occurs with formation of APAP-protein adducts. These adducts are formed by hepatic metabolism of APAP to N-acetyl-p-benzoquinone imine, which covalently binds to hepatic proteins as 3-(cystein-S-yl)-APAP adducts. Adducts are released into blood during hepatocyte lysis. We previously showed that adducts could be quantified by high-performance liquid chromatography with electrochemical detection following proteolytic hydrolysis, and that the concentration of adducts in serum of overdose patients correlated with toxicity. The following study examined the pharmacokinetic profile and clinical associations of adducts in 53 adults with acute APAP overdose resulting in acute liver failure. A population pharmacokinetic analysis using nonlinear mixed effects (statistical regression type) models was conducted; individual empiric Bayesian estimates were determined for the elimination rate constant and elimination half-life. Correlations between clinical and laboratory data were examined relative to adduct concentrations using nonparametric statistical approaches. Peak concentrations of APAP-protein adducts correlated with peak aminotransferase concentrations (r = 0.779) in adults with APAP-related acute liver failure. Adducts did not correlate with bilirubin, creatinine, and APAP concentration at admission, international normalized ratio for prothrombin time, or reported APAP dose. After N-acetylcysteine therapy, adducts exhibited first-order disappearance. The mean elimination rate constant and elimination half-life were 0.42 +/- 0.09 days(-1) and 1.72 +/- 0.34 days, respectively, and estimates from the population model were in strong agreement with these data. Adducts were detected in some patient samples 12 days post-ingestion. The persistence and specificity of APAP-protein adducts as correlates of toxicity support their use as specific biomarkers of APAP toxicity in patients with acute liver injury.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
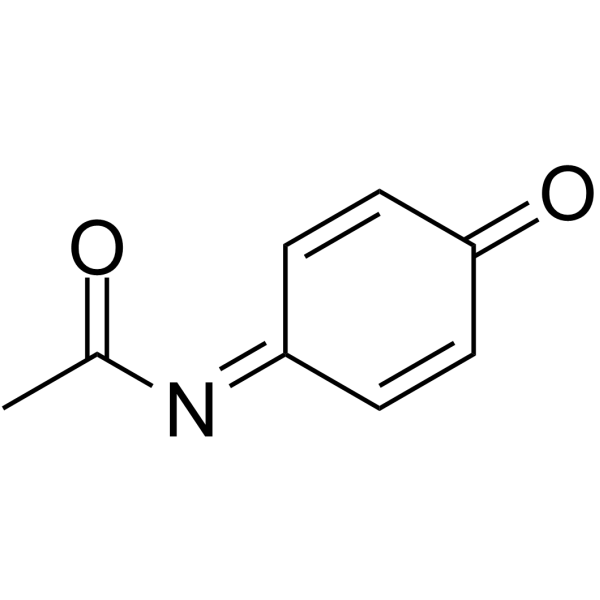 |
N-Acetylimidoquinone
CAS:50700-49-7 |
C8H7NO2 |
|
Targeting mitochondria with methylene blue protects mice aga...
2015-01-01 [Hepatology 61(1) , 326-36, (2015)] |
|
Monitoring paracetamol metabolism after single and repeated ...
2007-09-01 [Int. J. Clin. Pharmacol. Ther. 45(9) , 496-503, (2007)] |
|
Use of a systems model of drug-induced liver injury (DILIsym...
2014-04-21 [Toxicol. Lett. 226(2) , 163-72, (2014)] |
|
In-source formation ofN-acetyl-p-benzoquinone imine (NAPQI),...
2011-01-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 879(17-18) , 1476-84, (2011)] |
|
Combination of electrochemistry and nuclear magnetic resonan...
2012-10-16 [Anal. Chem. 84(20) , 8777-82, (2012)] |