A catalyst-free multicomponent domino sequence for the diastereoselective synthesis of (E)-3-[2-arylcarbonyl-3-(arylamino)allyl]chromen-4-ones.
Pitchaimani Prasanna, Pethaiah Gunasekaran, Subbu Perumal, J Carlos Menéndez
Index: Beilstein J. Org. Chem. 10 , 459-65, (2014)
Full Text: HTML
Abstract
The three-component domino reactions of (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones, 3-formylchromone and anilines under catalyst-free conditions afforded a library of novel (E)-3-(2-arylcarbonyl-3-(arylamino)allyl)-4H-chromen-4-ones in good to excellent yields and in a diastereoselective transformation. This transformation generates one C-C and one C-N bond and presumably proceeds via a reaction sequence comprising a Michael-type addition-elimination reaction, a nucleophilic attack of an enamine to a carbonyl reminiscent of one of the steps of the Bayllis-Hilman condensation, and a final deoxygenation. The deoxygenation is assumed to be induced by carbon monoxide resulting from the thermal decomposition of the dimethylformamide solvent.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
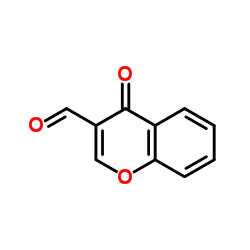 |
4-Oxo-4H-chromene-3-carbaldehyde
CAS:17422-74-1 |
C10H6O3 |
|
Formylchromone exhibits salubrious effects against nitrosodi...
2014-08-05 [Chem. Biol. Interact. 219 , 175-83, (2014)] |
|
3-Formylchromones: potential antiinflammatory agents.
2010-09-01 [Eur. J. Med. Chem. 45 , 4058-64, (2010)] |
|
Synthesis of quinolines from 3-formylchromone.
2008-08-01 [J. Org. Chem. 73(15) , 6010-3, (2008)] |
|
Loss of H2 and CO from protonated aldehydes in electrospray ...
2014-09-15 [Rapid Commun. Mass Spectrom. 28(17) , 1871-82, (2014)] |
|
Thermal solvent-free synthesis of chromonyl chalcones, pyraz...
2012-02-01 [J. Enzyme Inhib. Med. Chem. 27(1) , 84-91, (2012)] |