Excited-state prototropic equilibrium dynamics of 6-hydroxyquinoline encapsulated in microporous catalytic faujasite zeolites.
Sun-Young Park, Hyunung Yu, Jiho Park, Du-Jeon Jang
Index: Chemistry 16(42) , 12609-15, (2010)
Full Text: HTML
Abstract
The excited-state proton transfer and geminate recombination of 6-hydroxyquinoline (6HQ) encaged in catalytic Na(+)-exchanged faujasite zeolites X (NaX) and Y (NaY) have been explored by measuring steady-state and picosecond time-resolved spectra. The pathways and rate constants of proton transfer of excited 6HQ are determined by the microscopic environment of zeolitic hosts surrounding the guest molecules. The excited-state proton transfer of a 6HQ molecule encapsulated in a zeolitic nanocavity is initiated by deprotonation of the enolic group to form an anionic intermediate and completed by subsequent protonation of the imino group to form a zwitterionic tautomer. Geminate recombination occurs to compete with proton transfer at each tautomerization step of excited-state 6HQ because of the confined environment of dehydrated zeolitic supercages. Consequently, excited-state equilibria among three prototropic species of 6HQ are established in microporous catalytic faujasite zeolites. Kinetic differences in NaX and NaY are attributed to dissimilarities in acidity/basicity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
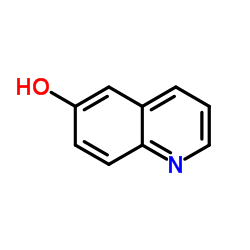 |
6-Hydroxyquinoline
CAS:580-16-5 |
C9H7NO |
|
Synthesis and antimosquito properties of 2,6-substituted ben...
2013-07-01 [Eur. J. Med. Chem. 65 , 295-303, (2013)] |
|
TDDFT study of the polarity controlled ion-pair separation i...
2014-07-15 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 128 , 280-4, (2014)] |
|
Excited state proton transfer in the Cinchona alkaloid cupre...
2010-10-21 [Phys. Chem. Chem. Phys. 12(39) , 12562-9, (2010)] |
|
Excited-state proton transfer via hydrogen-bonded acetic aci...
2011-01-13 [J. Phys. Chem. A 115(1) , 19-24, (2011)] |
|
Proton translocation and electronic relaxation along a hydro...
2008-07-17 [J. Phys. Chem. B 112(28) , 8383-6, (2008)] |