Excited state proton transfer in the Cinchona alkaloid cupreidine.
Junhong Qian, Albert M Brouwer
Index: Phys. Chem. Chem. Phys. 12(39) , 12562-9, (2010)
Full Text: HTML
Abstract
Photophysical properties of the organocatalyst cupreidine (CPD) and its chromophoric building block 6-hydroxyquinoline (6HQ) in protic and nonprotic polar solvents (methanol and acetonitrile) were investigated by means of UV-vis absorption, and steady state and time resolved fluorescence spectroscopy. The effects of the catalytically relevant interactions with electrophilic and hydrogen bonding agents (p-toluene sulfonic acid and water) on their spectral characteristics were studied. In neutral CPD in acetonitrile, quenching of fluorescence occurs due to electron transfer from the quinuclidine nitrogen to the excited quinoline chromophore. Protonation suppresses this process, while complexation with water leads to enhanced excited state proton transfer from the 6'-OH group to the quinuclidine nitrogen, and emission occurs from the anionic form of the chromophore. The weakly emitting zwitterionic form of the hydroxyquinoline chromophore is readily formed in methanol, but not in acetonitrile.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
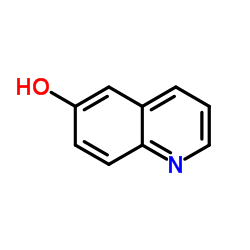 |
6-Hydroxyquinoline
CAS:580-16-5 |
C9H7NO |
|
Synthesis and antimosquito properties of 2,6-substituted ben...
2013-07-01 [Eur. J. Med. Chem. 65 , 295-303, (2013)] |
|
TDDFT study of the polarity controlled ion-pair separation i...
2014-07-15 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 128 , 280-4, (2014)] |
|
Excited-state prototropic equilibrium dynamics of 6-hydroxyq...
2010-11-08 [Chemistry 16(42) , 12609-15, (2010)] |
|
Excited-state proton transfer via hydrogen-bonded acetic aci...
2011-01-13 [J. Phys. Chem. A 115(1) , 19-24, (2011)] |
|
Proton translocation and electronic relaxation along a hydro...
2008-07-17 [J. Phys. Chem. B 112(28) , 8383-6, (2008)] |