Thermochromism of heme adducts of Glycera hemoglobin and some other monomeric heme proteins.
J J Stephanos, A W Addison
Index: J. Inorg. Biochem. 39(4) , 351-69, (1990)
Full Text: HTML
Abstract
The thermally induced difference spectra of myoglobin (Mb) and Glycera dibranchiata hemoglobin (Hbm) derivatives and of cytochrome-c were recorded between 4 degrees and 30 degrees C in the 390-750 nm range. Thermodynamic parameters were estimated and upper and lower temperature limiting spectra were deduced for the various heme protein derivatives' equilibria. The effective iron d-electron population divides the hemes broadly into two different groups of behavior type. In the first group, Hbm(III)N3, Hbm(III), Mb(III)(H2O), and Cytc(III) show equilibria between two spin states. The weakest coupling between the heme and the globin occurs among the second group, for Hbm(II)CO and Mb(II)CO, which in the higher temperature limit undergoes averaging of the carbonyl tilt, while an axially elongated geometry is probably accessed for Hbm(II)NO and Mb(II)NO. Examples of the less common situation of increased absorption intensity and/or low-spin states at higher temperature were found in both groups. In the case of the methyl thioglycolate low-spin adducts of Hbm(III), an acid/base equilibrium involving thioglycolate deprotonation occurs. Apparent enthalpy-entropy compensation is exhibited by all these heme derivatives, and it is suggested that the delta H degrees and delta S degrees values relate to the intimacy of coupling between the heme structure and the solvent-dependent microconformation of the globin.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
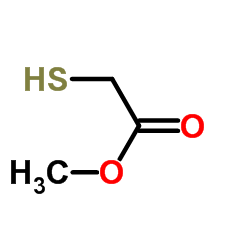 |
Methyl thioglycolate
CAS:2365-48-2 |
C3H6O2S |
|
Blood-brain barrier permeable gold nanoparticles: an efficie...
2014-12-29 [Small 10(24) , 5137-50, (2014)] |
|
Ligand-controlled growth of ZnSe quantum dots in water durin...
2012-09-11 [Langmuir 28(36) , 12931-40, (2012)] |
|
Simultaneous determination of dimethylarsinic acid and monom...
1999-01-01 [Rapid Commun. Mass Spectrom. 13(5) , 350-3, (1999)] |
|
Gold Nanorods Conjugated with Doxorubicin and cRGD for Combi...
2012-01-01 [Theranostics 2(8) , 757-68, (2012)] |
|
Arylalkylidene rhodanine with bulky and hydrophobic function...
2001-01-22 [Bioorg. Med. Chem. Lett. 11(2) , 91-4, (2001)] |