Pentacyclic polyketides from Endiandra kingiana as inhibitors of the Bcl-xL/Bak interaction.
Aurélie Leverrier, Khalijah Awang, Françoise Guéritte, Marc Litaudon
Index: Phytochemistry 72(11-12) , 1443-52, (2011)
Full Text: HTML
Abstract
An in vitro biological screening of Malaysian plants allowed the selection of several species with a significant binding affinity for the antiapoptotic protein Bcl-xL. The chemical investigation of Endiandra kingiana led to the isolation of a series of polyketides named kingianins A-N, having a pentacyclic carbon skeleton described for the first time in nature. Fourteen compounds were isolated as racemic mixtures, and characterized by mass spectrometryand extensive one- and two-dimensional NMR spectroscopy. The (-) and (+) enantiomers of kingianins A and G-L were separated using chiral HPLC, and the absolute configuration of four of them was clearly established by CD analysis. The levorotatory enantiomers showed the more potent binding affinity for Bcl-xL with Ki ranging from 1.0 to 12μM.Copyright © 2011 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
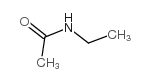 |
N-ethylacetamide
CAS:625-50-3 |
C4H9NO |
|
Shear and dielectric responses of propylene carbonate, tripr...
2012-08-14 [J. Chem. Phys. 137(6) , 064508, (2012)] |
|
Acetyl Methyl Torsion in N-Ethylacetamide: A Challenge for M...
2015-06-22 [ChemPhysChem 16 , 1906-11, (2015)] |
|
The effect of anesthetics on hydrogen bonds. An infrared stu...
1985-10-01 [Biophys. Chem. 22(4) , 249-54, (1985)] |
|
Identification of dielectric and structural relaxations in g...
2005-08-01 [J. Chem. Phys. 123(5) , 054516, (2005)] |
|
Fractional Stokes-Einstein-Debye relation and orientational ...
2011-03-07 [Phys. Chem. Chem. Phys. 13(9) , 3911-6, (2011)] |